Vehicles used for parenteral preparations should possess the following characteristics:
- They should be well tolerated by the body.
- They should be easy to handle during formulation.
- They should be capable of dissolving the drug.
- They should be sterile and pyrogen-free.
- They should have optimum viscosity.
Vehicles used for parenteral preparations are classified as follows:
- Aqueous vehicles
- Nonaqueous vehicles
Aqueous Vehicles
- Water for Injection USP:Figure 8.2 shows the water for injection flow chart:
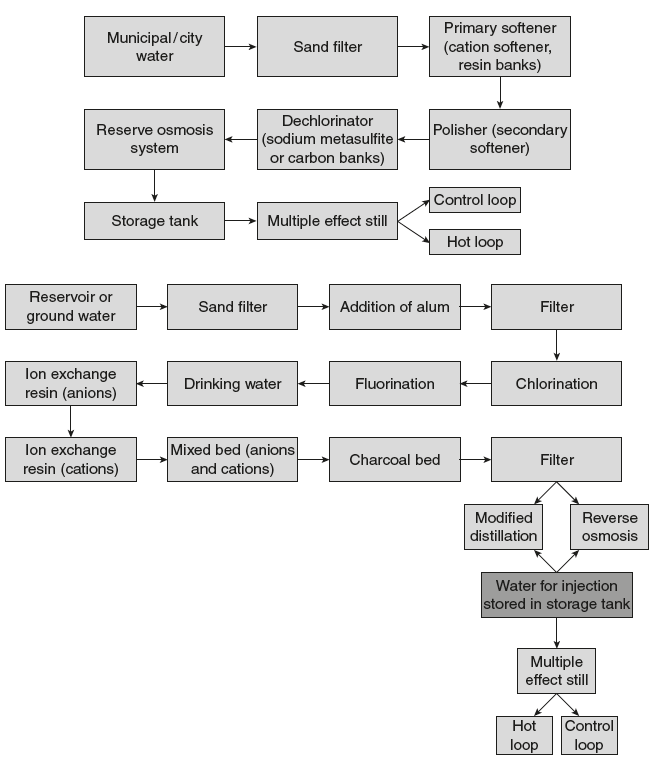 Figure 8.2 Preparation of Water for Injection
Figure 8.2 Preparation of Water for Injection
- The most frequently used solvent in the large-scale manufacturer of injections is water for injection USP.
- This water is purified by distillation or by reverse osmosis and meets the same standards for the presence of total solids as that of purified water, which is not more than 1 mg/100 ml of water for injection USP, and may not contain added substances.
- Although water for injection is not required to be sterile, it must be pyrogen-free.
- This water can be used in the manufacture of injectable products that are terminally sterilized after preparation.
- Water for injection is intended to be used within 24 hours after collection. It should be collected in sterile and pyrogen-free containers.
- Sterile Water for Injection USP:
- Sterile water for injection USP is packaged in single-dose containers not exceeding 1 liter capacity.
- It must be pyrogen-free and should have an allowable endotoxin level, which is not more than 0.25 USP endotoxin units per milliliter.
- It should not contain any antimicrobial agent or other added substance.
- This water may contain slightly more total solids than water for injection because of the leaching of solids from the glass-lined tanks during sterilization.
- This water is intended to be used as a vehicle for already sterilized and packaged injectable medications.
- This water is used for reconstitution of antibiotics. It is aseptically added to the vial of medication to prepare the desired injection. For instance, a suitable injection may be prepared from the sterile dry powder ampicillin sodium USP by aseptic addition of sterile water for injection.
- Bacteriostatic Water for Injection USP:
- Bacteriostatic water for injection USP is sterile water for injection containing one or more suitable antimicrobial agents.
- It is packaged in prefilled syringes or in vials containing not more than 30 ml of the water.
- The label must state the names and proportions of the antimicrobial agents.
- This water is employed as a sterile vehicle in small volume parenterals.
- Bacteriostatic agents are preferred for multiple-dose parenterals.
- Because of the presence of antimicrobial agents, this water must be used only in parenterals that are administered in small volumes.
- Bacteriostatic agent or agents must be chemically compatible with the particular medicinal agent being dissolved or suspended.
- This type of water should not be used in neonates.
- Sodium Chloride Injection USP:
- Sodium chloride injection USP is a sterile isotonic solution of sodium chloride in water for injection.
- It contains no antimicrobial agents but has approximately 154 mEq each of sodium and chloride ions per liter.
- It may be used as a sterile vehicle in solutions or suspensions of drugs for parenteral administration.
- It is frequently used as a catheter or intravenous line flush. Catheters or intravenous lines are constantly used to infuse fluids and intravenous medications and draw blood for laboratory analysis. Usually, 2 ml is used to flush the line after each use or every eight hours if the line is not used.
- Bacteriostatic Sodium Chloride Injection USP:
- Bacteriostatic sodium chloride injection USP is a sterile isotonic solution of sodium chloride in water for injection.
- It contains one or more suitable antimicrobial agents, which should be mentioned on the label.
- Sodium chloride 0.9% renders the solution isotonic.
- When this solution is used as a vehicle, care must be exercised to ensure compatibility of the drug with the preservative and sodium chloride.
- It can also be used to flush a catheter or intravenous line to maintain its potency.
- It should not be used in neonates.
- Ringer’s Injection USP:
- Ringer’s injection USP is a sterile solution of sodium chloride, potassium chloride, and calcium chloride in water for injection.
- The three agents are present in concentrations similar to those of physiologic fluids.
- Ringer’s is employed as a vehicle for other drugs or alone as an electrolyte replenisher and plasma volume expander.
- Lactated Ringer’s injection USP is a sterile solution of sodium chloride, potassium chloride, calcium chloride and sodium lactate in water for injection.
- This injection is used as a fluid and electrolyte replenisher and as systemic alkalizer.
Leave a Reply