Learning Objective
- Various quality control tests used for sterile dosage forms
Leak Test
Sealing of ampoules may be done by tip sealing and pull sealing methods. During the sealing process there are chances that ampoules are incompletely sealed and cracks may occur around the seal or at the base of the ampoule as a result of improper handling. Hence, the leak test is intended to detect improperly filled and sealed ampoules so that they can be discarded.
The test is performed by immersing the sample ampoules in a deeply colored dye solution (usually 0.5% to 1.0% methylene blue) and by applying a negative pressure in a vacuum chamber with 27 inches Hg or more for 30 minutes. After the test period, the vacuum is gently released and the subsequent atmospheric pressure causes the dye to penetrate an opening if present, being visible after the ampoules have been washed externally and visually observed.
During the cycles of autoclaving the leak test can be performed by immersing the ampoules in a bath of dye. This provides an advantage of achieving both leak detection and sterilization in one process. Vials and bottles are not subjected to such a leak test because the rubber closure is not rigid.
Clarity Test
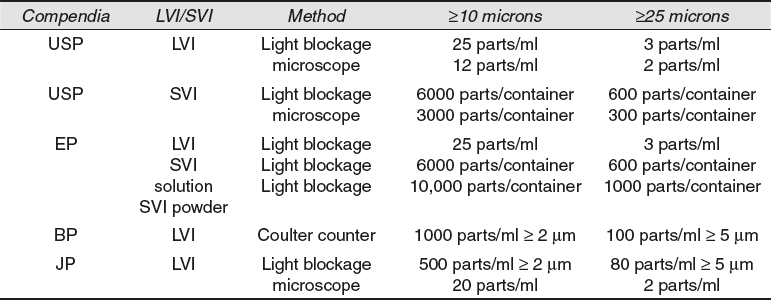
Clarity test is performed to confirm the quality and purity of sterilized parenteral solutions. Sometimes, these solutions contain visible particulate matter ranging from 30 μm to 40 μm and larger in size.
The USP states that GMP requires that all containers be visually inspected and that any contained with visible particles be discarded. In addition, for large volume infusions, the USP has established a limit of 50 particles of 10 microns and larger and 5 particles of 25 microns and larger (Table 8.5). Although particulate matter is of primary concern in products given intravenously, all parenteral products should be free from insoluble particles.
The visual inspection of a product container is usually done by individual human inspection of each externally clean container with the contents set in motion with a swirling action, under a good light, baffled against reflection into the eyes and viewed against a black and white background. A moving particle is much easier to see than a stationary one but care must be taken to avoid introducing air bubbles, which are difficult to distinguish from particulate matter. To see heavy particles, it may be necessary to invert the container as the final step during inspection.
Evaluation of particulate matter in liquids involves instrumental methods, which involves utilization of principles such as light scattering, light absorption and electrical resistance to obtain particle count and size distribution.
Table 8.5 Clarity Test Official Standards
Drug Content
Unless otherwise stated in the individual monograph, suspensions for injection that are presented in single-dose containers and that contain less than 10 mg or less than 10% of active ingredient comply with the following test.
The test is performed by randomly selecting 10 samples, which are estimated for drug content using the method given in the monograph or by any other suitable analytical method. The test passes if the result complies between 85% and 115% of the average value. The preparation under examination fails to comply with the test if more than one individual value is outside the limits 85%–115% of the average value or if any one individual value is outside the limits 75%–125% of the average value.
If one individual value is outside the limits of 85%–115% but within the limits 75%–125% of the average value, the determination is repeated using another 20 containers taken at random. The preparation under examination complies with the test if in the total sample of 30 containers, not more than one individual value is outside the limits 85%–115% and none is outside the limits 75%–125% of the average value.
Note: The test for uniformity of content is not applicable to suspensions for injection containing multivitamins and trace elements.
Pyrogen Test
The endotoxin metabolites of microorganisms that cause marked rise in the body temperature when contaminated with the parenteral preparations are called as pyrogens. The tests for pyrogens are carried out as follows:
Rabbit Test
The USP pyrogen test uses healthy rabbits that have been properly maintained in terms of environment and diet before the test. Normal or control temperatures are taken for each animal to be used in the test. These temperatures are used as the base for the determination of any temperature increase resulting from injection of the test solution. A given test uses three rabbits whose temperatures do not differ by more than 1°C from each other and whose body temperatures are considered not to be elevated. The product to be tested is warmed to 37°C ± 2°C and injected into the marginal ear vein of each of rabbits, completing each injection within 10 minutes of the start of administration. Temperature should be recorded at 30-minute intervals up to 1–3 hours subsequent to the injection. If no rabbit shows an individual rise in temperature of 0.5°C or more, the product meets the requirements for the absence of pyrogens. If any rabbit shows an individual temperature rise of 0.5°C or more, the test should be continued using five other rabbits. If not more than three of the eight rabbits show individual rises in temperature of the material under examination, it meets the requirements for the absence of pyrogens.
Limulus Amebocyte Lysate Test
The limulus amebocyte lysate (LAL) test is the in vitro test method for pyrogens that has been developed utilizing the gelling property of the lysate of the amebocyte of Limulus polyphemus (the horseshoe crab). In the presence of pyrogenic endotoxins from Gram-negative bacteria, a firm gel is formed within 60 minutes, when incubated at 37°C. The LAL test has been found to be 5 to 10 times more sensitive than the rabbit test and by the use of serial dilutions, it has been shown to be semiquantitative.
Sterility Test
This is a confirmatory test for the procedure of sterilization. Preparations and materials such as injectables, ophthalmic products and absorbent cotton that are required to be sterile are tested. The test is carried out under aseptic conditions to avoid contamination of the product during the test.
Leave a Reply