Learning Objective
- Types of chemical incompatibility
Mixtures with chemical incompatibilities are as follows:
- Alkaloidal salts with alkaline substances: Alkaloids are weak bases which are slightly soluble or insoluble in water but alkaloidal salts are soluble in water. If these salts are dispensed with alkaline preparations such as strong ammonia solution or ammonium bicarbonate, the free alkaloid may be precipitated out. Example:Strychnine hydrochloride solution5 mlAromatic spirit of ammonia3.332 mlWater (q.s.)100 ml Incompatibility: Strychnine hydrochloride is an alkaloidal salt, whereas aromatic spirit of ammonia is an alkaline substance. When they both react, strychnine gets precipitated because the quantity of strychnine hydrochloride prescribed is more than its solubility in water. This preparation contains negligible amount of alcohol that cannot dissolve strychnine. Hence, it gets precipitated as diffusible precipitate.Remedy: Divide the vehicle into two portions. The reactants are dissolved in separate portions and mixed slowly by adding one to the other with rapid stirring. The formed diffusible precipitates are uniformly dispersible with mild shaking of the contents.
- Alkaloidal salts with salicylates Example:Quinine hydrochloride1.2 gSodium salicylate2.4 gWater (q.s.)100 ml Incompatibility: When quinine compounds are combined with salicylates, indiffusible precipitates of quinine salicylate are formed.Remedy: Divide the vehicle into two portions. The first reactant is dissolved in the first portion.Suitable amount of compound tragacanth powder is weighed (2 g/100 ml of the finished product) into a mortar and triturated with the second portion of the vehicle to form smooth mucilage. The second reactant is dissolved in this mucilage and adjusted to suitable volume. The first mixture is then slowly added to the second mixture with rapid stirring.
- Soluble salicylates with ferric salts Example:Ferric chloride solution2 mlSodium salicylate3 gWater (q.s.)100 ml Incompatibility: Ferric salts react with sodium salicylate to liberate indiffusible precipitates of ferric salicylate.Remedy: Divide the vehicle into two portions. The first reactant is dissolved in the first portion.Suitable amount of compound tragacanth powder is weighed (2 g/100 ml of the finished product) into a mortar and triturated with the second portion of the vehicle to form the smooth mucilage. The second reactant is dissolved in this mucilage and adjusted to suitable volume. The first mixture is then slowly added to the second mixture with rapid stirring. (or) Sodium bicarbonate is added to the preparation. In the presence of sodium bicarbonate, the precipitates of sodium salicylate remain soluble to form a clear mixture.
- Soluble salicylates with alkali bicarbonates Example:Sodium salicylate10 gSodium bicarbonate4gChloroform water (q.s.)100 ml Incompatibility: If sodium salicylate solutions are dispensed with alkaline substances such as sodium bicarbonate, the mixture undergoes oxidation (by absorbing oxygen) and turns reddish brown. This does not change the therapeutic efficacy of the mixture, but may lead to anxiety in the patient.Remedy: A dark coloring agent like liquorice liquid extract may be added. (or) An antioxidant such as sodium metabisulfite (0.1% w/v) can be added to prevent oxidation.
- Soluble salicylates and benzoates with acidsMost acids and acid syrups decompose sodium salicylate or sodium bezoate to form precipitates of salicylic acid and benzoic acid, respectively. Example:Sodium salicylate5.01 gLemon syrup25 mlWater (q.s.)100 ml Incompatibility: Lemon syrup contains citric acid. When it reacts with sodium salicylate, indiffusible precipitates of salicylic acid are formed.Remedy: Divide the vehicle into two portions. The first reactant is dissolved in the first portion.Suitable amount of compound tragacanth powder is weighed (2 g/100 ml of the finished product) into a mortar and triturated with the second portion of the vehicle to form smooth mucilage. The second reactant is dissolved in this mucilage and adjusted to suitable volume. The first mixture is then slowly added to the second mixture with rapid stirring. (or) Replace lemon syrup with a mixture of plain syrup and tincture of lemon.
- Incompatibility leading to evolution Of CO2When carbonates or bicarbonates and acidic drugs are dispensed in a mixture along with water, they react together leading to the evolution of CO2.Remedy: To prevent container leakage or explosion, the reaction must be completed before the preparation is transferred to the container. The ingredients are mixed in an open vessel and the effervescence reaction is allowed to complete after which it is transferred. Example:Sodium bicarbonate4 gBorax2 gGlycerol20 mlWater (q.s.)100 ml Incompatibility: When borax and glycerol are mixed together, hydrolysis of borax takes place with the formation of boric acid.
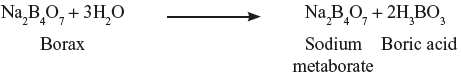 Boric acid reacts with glycerol to form monobasic glyceryl boric acid.
Boric acid reacts with glycerol to form monobasic glyceryl boric acid.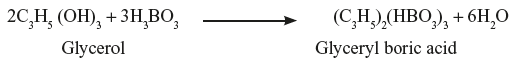 The glyceryl boric acid further reacts with sodium bicarbonate to evolve CO2.
The glyceryl boric acid further reacts with sodium bicarbonate to evolve CO2.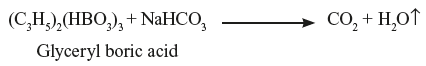 Remedy: All the ingredients are mixed effervescence is allowed to take place and if needed slightly warmed. Once it stops, it is transferred to the container.
Remedy: All the ingredients are mixed effervescence is allowed to take place and if needed slightly warmed. Once it stops, it is transferred to the container. - Herapathite reaction (quinine sulfate with iodides) Example:Quinine sulfate5 gDilute sulfuric acid10 mlPotassium iodide1.5 gWater (q.s.)100 ml Incompatibility: Quinine sulfate is not freely soluble in water. It is made soluble in the presence of dilute sulfuric acid. The sulfuric acid liberates hydroiodic acid from the potassium iodide. The hydroiodic acid is partly oxidized by sulfuric acid, yielding iodine.The iodine, hydroiodic acid and quinine sulfate then combine to form a compound called “herapathite or iodosulfate of quinine”. The mixture formed is quite clear at first but after about three days, it may deposit bronze or olive green scales, which are due to “herapath reaction for quinine”.Herapath reaction:
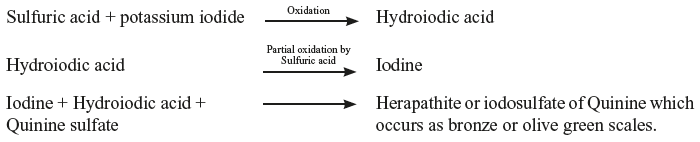 Remedy: To avoid any problems, it should be given to the patient only for about three days. (or) The mixture should be divided, sending the potassium iodide in one bottle and quinine sulfate in another bottle. The patient should be advised to mix both the solutions and take the necessary dose.
Remedy: To avoid any problems, it should be given to the patient only for about three days. (or) The mixture should be divided, sending the potassium iodide in one bottle and quinine sulfate in another bottle. The patient should be advised to mix both the solutions and take the necessary dose. - Soluble iodides with potassium chlorate Example:Potassium chlorate2.22 gSyrup of ferric iodide27.8 mlWater (q.s.)100 ml Incompatibility: The ferric iodide is oxidized by potassium chlorate and the reaction is as follows: KClO3 + 3FeI3 → 3FeOI + 3I2 + KCl Remedy: The mixture is clear when freshly prepared but deposits crystals of iodine upon storage for sometime. So, the two reacting substances must be dispensed in separate bottles with a label indicating “Mix the contents of both the bottles before use”.
- Potassium chlorate and oxidizable substances (Explosive mixture): Example:Potassium chlorate0.6 gTannic acid0.3 gSucrose0.3 g Incompatibility: When potassium chlorate (oxidizing agent) is triturated or heated with readily oxidizable substances (reducing agents) such as charcoal, sulfur or tannic acid, there are chances of an explosion.Remedy: Potassium chlorate and tannic acid are triturated individually. Then the powders are mixed separately with half the quantities of powdered sucrose and finally they are mixed together lightly using a spatula.
Leave a Reply