The term tertiary structure refers to the unique three-dimensional confirmations that globular protein assumes as a consequence of the interaction between the side chains in their primary structure.
Tertiary structure has several important features.
The amino acids that are distant from each other in the primary structure come closer during the formation of the tertiary structure. As a result of this property, the polypeptide chain folds in a compact manner. During this process, most water molecules are excluded from the protein’s interior, making interactions between both polar and non-polar groups.
Large globular proteins (i.e. those with more than two hundred amino acid residues) often contain several compact units called domains. Domains are typically structurally independent segments that have specific functions (e.g.: binding an ion on small molecule).
The following three domains are found in several proteins:
The EF hand, which consists of a helix–loop–helix configuration, binds specifically to Ca2+ as shown in Figure 3.10.
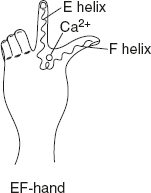
Figure 3.10 Structure of Helix-loop-helix Pattern
The zinc finger motif is commonly found in DNA-binding proteins as shown in Figure 3.11. Zinc finger promotes protein DNA interaction.
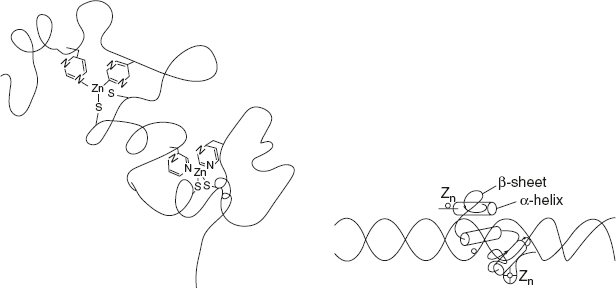
Figure 3.11 Structure of Zinc Finger Motif
Leucine zipper – DNA-binding domain as shown in Figure 3.12.
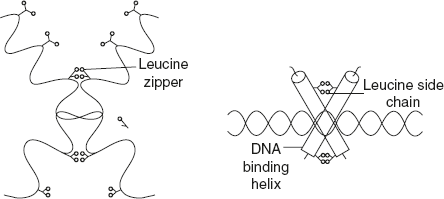
Figure 3.12 Structure of Leucine Zipper Motif
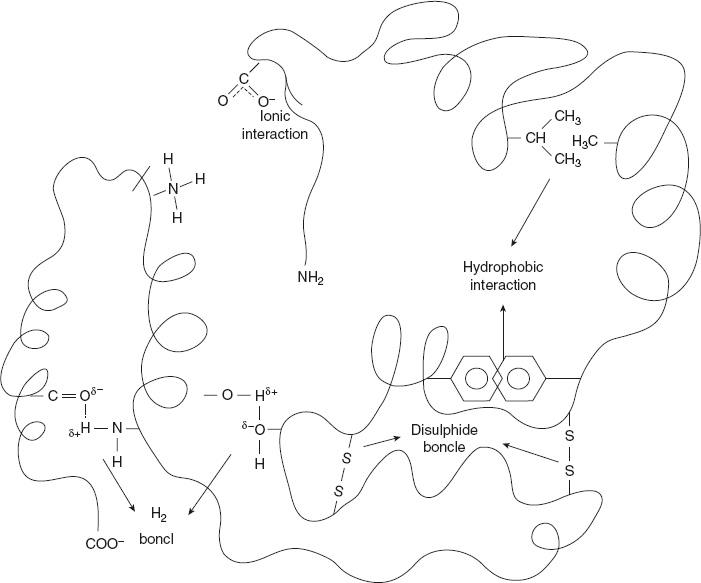
The following type of interaction stabilises the tertiary structure:
Leave a Reply