In fibrous proteins, bundles of helical polypeptide are commonly twisted together into larger bundles. The structural unit of the α-keratins—a class of proteins found in the hair, wool, skin, horns, and finger nails is an α-helical polypeptide. Each peptide has three domains: an amino terminal head domain, a central rod-like α-helical domain, and a carboxyl terminal tail.
There are two families of α-keratin polypeptides: type 1 and type 2. One peptide from each family associates to form a coiled coil dimer. Two anti-parallel rows of theses dimers form a supercoiled structure called protofilament. Disulphide bridges and hydrogen bonds are the basic interactions between protofilaments. Four protofilaments are packed together to form hundreds of filament. All the filaments collectively are called as macrofilaments. The hair cell, also called a fibre, is made up of several microfibrils, as shown in Figure 3.16.
The α-helical rod-like domains of a type 1 polypeptide and type 2 polypeptide form a coiled coil. Two staggered anti-parallel rows of these dimers form a supercoiled protofilament. Hundreds of filaments, each containing four protofilaments, form a microfibril.
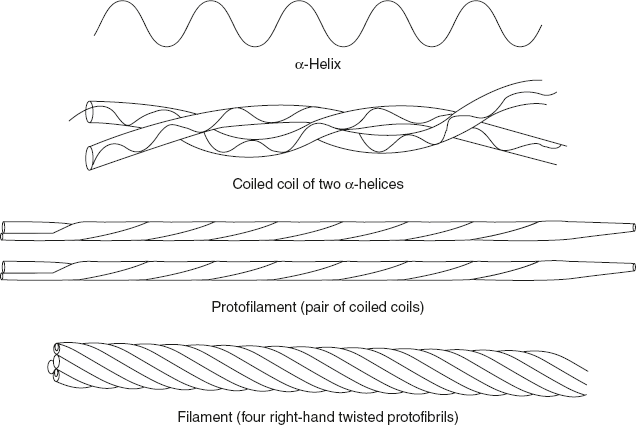
Figure 3.16 Diagrammatic Representation of α-keratin Structure
Leave a Reply