Mitochondrial ATP synthase is an F-type ATPase. It catalyses the formation of ATP and Pi accompanied to the flow of protons from the P to N side of the membrane. ATP synthase also called complex V has two distinct components:
F1 – a peripheral membrane protein
F0 – integral to the membrane, and 0 stands for oligomycin sensitive
Efraim Racker first identified this enzyme.
F1 has nine subunits of five different types, with the composition α3β3γδε. Each of the three β subunits has one catalytic site for ATP synthesis. John E Walker determined the F1 structure.
The knob-like portion of F1 consists of alternating α and β subunits arranged like sections of an orange.
The polypeptide that makes up the stalk in the F1 crystal structure is asymmetrically arranged with one domain of the single γ subunit, making up a central shaft that passes through F1 and another domain of γ associated primarily with one of the three β subunits.
The F0 complex, making up the proton pore, is composed of three subunits. a, b, and c in the proportion ab2c10. Subunit c is a small very hydrophobic polypeptide, consisting almost entirely of two transmembrane helices with a small loop extending from the matrix side of the membrane. The γ ε subunits of F1 form a leg and foot that project from the bottom side of F1 and stand as firm rings of (subunits as shown in Figure 8.21).
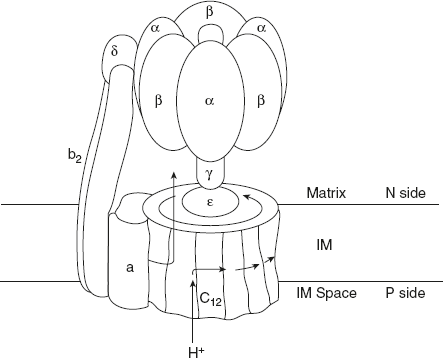
Figure 8.21 Structure of ATP synthase
Leave a Reply