Rotational catalysis a key to the binding change mechanism for ATP synthesis. Paul Boyer proposed a mechanism in which the three active sites of F1 take turns in the catalysis of ATP synthesis. A given β subunits start in the β-ADP conformation, which binds ADP and Pi from the surrounding medium. The subunit now changes conformation, assuming the β subunit changes to form that tightly binds and stabilises ATP. Finally, the subunit changes to β empty conformation, which has low affinity for ATP, and the newly synthesised ATP leaves the enzyme surface.
There is another round of analysis when this subunit again assumes the β-ADP forms and binds ADP and Pi. The conformational changes central to this mechanism are driven by the passage of protons through F0 portion of ATP synthase. The streaming of protons through F0 pore causes the cylinder of c subunits and the attached γ subunit to rotate about the long axis of γ, which is similar to the plane of membrane. The γ subunit passes through the centre of α3β3 spheroid. With each rotation of 1200, γ comes into contact with a different β empty conformation.
The three β subunits interact in such a way that the first subunit assumes the empty configuration, the second subunit assumes the β-ADP form, and the third subunit the β-ATP form. Thus one complete rotation of the γ subunit causes each subunit to cycle through all the three possible conformations. Moreover, for each rotation, three ATPs are synthesised and released from the enzyme surface. The mode of operation of ATP synthase is shown in Figure 8.22.
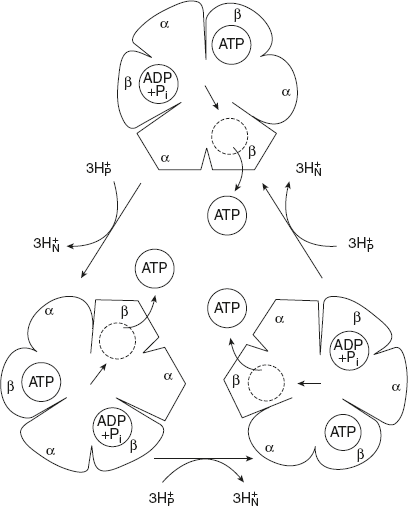
Figure 8.22 Binding-change Model for ATP Synthase
Leave a Reply