A pure preparation of a protein is essential, before studying its properties, amino acid composition and amino acid sequencing. The source of a protein is generally tissue or microbial cells. The first step in purifications is to open the cells and to release the proteins into a solution known as a crude extract. Next, differential centrifugation is carried out to collect the subcellular fractions. The subcellular fractions are now available for purifying one or more of the proteins present in them. Based on the size and charge, the proteins are separated and this process is known as fractionation. The solubility of protein is generally lowered at high salt concentrations—an effect called ‘salting-out’. The addition of salt in right amounts can selectively precipitate some proteins, while others remain in solution. Ammonium sulphate (NH4)2 SO4 is often used for this purpose because of its high solubility in water.
The most appropriate method for fractionating the proteins makes use of column chromatography as shown in Figure 3.20. Here, the proteins are separated based on their charge, size, binding affinity, and other properties. A stationary phase with porous solid material is packed in the column, and a mobile phase (buffer solution) is percolated through the column. Proteins in the eluents are placed on the top of the column and then percolated through the solid matrix. Proteins in the solutions migrate quickly or slowly based on their size and charge.
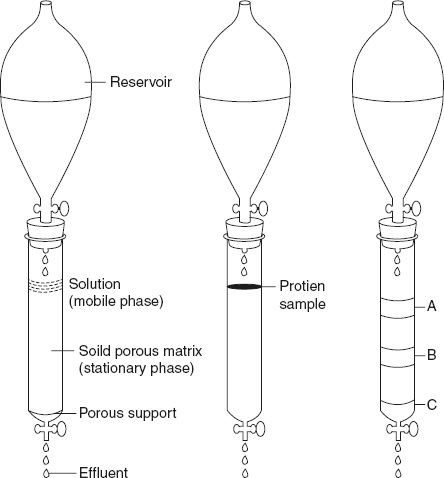
Figure 3.20 Column Chromatography
For example, in cation exchange chromatography, the solid matrix contains negatively charged groups. Proteins with net positive charge in the mobile phase migrate through matrix more slowly than those with net negative charge; this is because the migration of the former is retarded by the stationary phase. Two bands can be located when two types of proteins are present in the sample. As the length of the column increases, the resolution of the proteins will improve.
Leave a Reply