When [S] is lesser than Km, the velocity of the reaction is roughly proportional to the substrate concentration. The rate of the reaction is said to be first order with respect to substrate.
When [S] is greater than Km, the velocity of the reaction is constant and is equal to Vmax. Now the rate of reaction is independent of substrate concentration and is said to be zero order with respect to substrate concentration is explained in Figure 6.13.
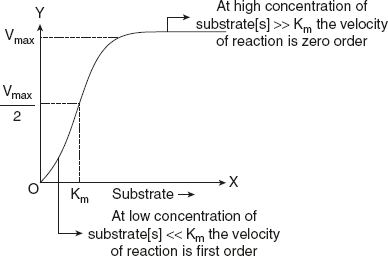
Figure 6.13 Graphical Representation of Order of Reaction with Related to Substrate Concentration
Leave a Reply