E + S → ES → E + P
E + I = EI


In the equal state,


[ET] = [E] + [ES] + [EI]
= [E] + [ES] +[E][I]/KI from (2)
= [E] + [E][I]/Ki +[ES]
= [E](1 + I/Ki) + [ES]
= Km[ES]/[S] (1 + I/Ki) + [ES] from (4)
ET = [ES](Km(1 + I/Ki)/[S] + 1)

For enzyme-catalysed reactions,



Substituting (8) in (5), we get,
Vmax/V = Km(1 + I/Ki)/[S] + 1
Vmax/V = Km(1 + I/Ki) + [S]/[S]
Vmax[S] = V(Km(1 + I/Ki)+[S]
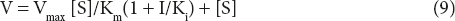

Where
![]() = Km(1 + I/KI)
= Km(1 + I/KI)
This is the M–M equation for competitive inhibitor.
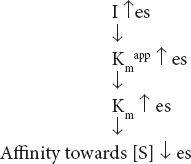
From the equation, it is clear that when an enzyme is inhibited competitively,
- Vmax does not change at all
- Km value increases as the concentration of I increases.
Leave a Reply