Salient featuresIn
this type of inhibitor, there is no structural similarity between I and S and hence no mutual competition between them for the active site of the enzyme.
The sites of attachment of I and S are different. Inhibitor does not bind with the active site of E but binds with a site other than the active site.
The non-competitive inhibitor binds with both free enzyme and ES complex. Thus, both EI and ESI complexes are formed as shown in Figure 6.21.
When I binds to E, it brings about three-dimensional structural changes in E, thus, inactivating it completely.
In this case, the extent of inhibition is independent of score because the percent of inhibitor is not reduced even by increasing the concentration of S. The combination with either S or I does not affect the affinity for the other.
Here Vmax is lowered, but Km is kept constant.
Chymotrypsin, where the catalytic site includes a proton acceptor, may be inhibited by increasing hydrogen conc.
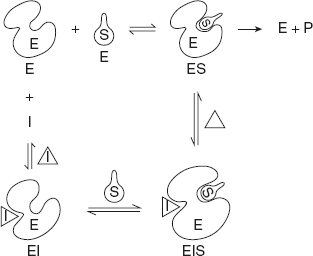
Figure 6.21 Non-competitive Inhibition
Leave a Reply