COMPETITIVE INHIBITION
The classical example for competitive inhibition is that of succinate dehydrogenase by malonate anion.
Succinate dehydrogenase catalyses the conversion of succinate to fumarate with FAD as cofactor. 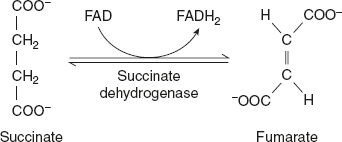
The enzyme catalyses the removal of two hydrogen atoms from succinate: one from each of the two methylene (—CH2) groups and FAD saves as hydride ion acceptor.
This enzyme is competitively inhibited by malonate, which resembles succinate in having two ionised carbonyl groups at pH 7.0 but differs in having only there carbon atoms.
However, malonate is not dehydrogenated by succinate dehydrogenase; it simply occupies the active site, keeping it away from acting on its normal substrate.
The catalytic site of succinate dehydrogenase has two approximately spaced positively charged groups capable of attracting the two negatively charged carboxylate group of succinate anion.
The effectiveness of malonate in competitively inhibiting succinate dehydrogenase is due to the complementarity between the active site of enzyme and its structure.
However, increasing the succinate concentration will reduce the extent of inhibitions by a given concentration of malonate (reversible).
Among other competitive inhibitors of succinate dehydrogenase having distactly placed anionic groups is oxaloacetate.
Leave a Reply