When the effector may be is different from substrate, in which case, the effector is said to be heterotrophic (Figure 6.29).
Example: Feedback inhibitors. Aspartate transcarbamoylase.
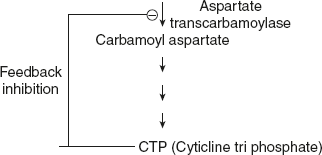
Figure 6.29 Heterotrophic Effector of Aspartate Transcarbamoylare
Conformational Changes in Allosteric Enzymes
Most of allosteric enzymes are oligomeric in nature. It exists in two conformational statuses: t (tense or taut) form and r (relaxed) form. The t– and r– states are in equilibrium. Allosteric inhibitors favour t-state, whereas activators and substrate favour r– state as shown in Figure 6.30.
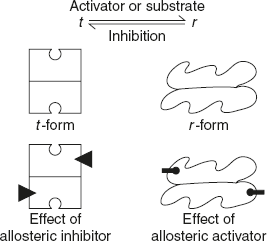
Figure 6.30 Allosteric Enzyme with t and r-state with Respect to the Activator and Inhibitor
Leave a Reply