The multiple forms of an enzyme catalysing the same reaction are isoenzymes or isozymes; they, however, differ in their physical and chemical properties, which include structure, electrophoretic and immunological properties, Km and Vmax values, optimum pH, relative susceptibility to inhibitors, and degree of denaturation.
Example: Lactate dehydrogenase LDH inter-converts the lactate and pyruvate (Figure 6.34).
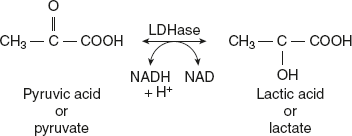
Figure 6.34 Conversion of Pyruvate to Lactate by Lactate Dehydrogenase
LDHase have five distinct isoenzymes. It is an oligomeric enzyme made up of four polypeptide subunits. Two types of subunits namely M (muscle) and H (heart) are produced by different genes. The five distinct isoenzymes LDH1, LDH2, LDH3, LDH4, and LDH5, can be separated by electrophoresis. LDH1 – more negative charge and LDH5 – more positive charge refer Figure 6.35.
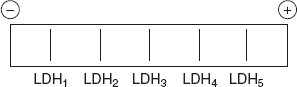
Figure 6.35 Electrophoritic Movement of LDH
| LDH1 (H4) |  | is predominantly found in heart muscle and is inhibited by pyruvate. Hence pyruvate is not converted to acetyl COA which enters TCA. |
| LDH2 (H3M) |  | is predominantly found in Heart and RBC. |
| LDH3 (H2M2) |  | is predominantly found in Brain and Kidney. |
| LDH4 (HM3) |  | is predominantly found in Liver and skeletal muscle. |
| LDH5 (M4) |  | is found in skeletal muscle and Liver. The inhibition of this enzyme by pyruvate is minimal and therefore pyruvate is converted to lactate. |
Leave a Reply