Saponins are high molecular weight, foam-forming glycosides based on the tetracyclic (steroidal) and pentacyclic tritepene groups and are characterized by their property of producing frothing aqueous solutions even at very low concentrations.
Detected in over 70 plant families, saponins occur as complex mixtures of glycosides, differing from one another in nature of sugar attachment or in the structures of the aglycone. The sugar may be glucose, galactose, glucoronic acid, xylose, rhamnose, or methyl pentose. The great complexity of saponin structure arises from the variability of the aglycone structure, the nature of the side chains, and the position of attachment of these moieties on the aglycone. Their glycosidic pattern is complex with as many as five sugar units attached and glucuronic acid is a common component. Saponins cause hemolysis when injected into the blood stream due to lysis of RBCs and increase in permeability of the plasma membrane. On account of their toxic nature, extracts of several saponin containing plants have been used as arrow poisons. However, they are harmless when taken orally due to low absorption and hydrolysis and plant foods such as lentils, beans, soyabeans, spinach, and oats are rich in saponins.
Steroidal alkaloids are nitrogen analogues of steroidal saponins and share their foaming and hemolytic property. However, they are toxic when ingested. Examples include solasodine and tomatidine from Solanum species.
On account of their surfactant properties and biological activities, saponins are in commercial demand with applications in the food, cosmetic, and pharmaceutical industries.
Plants rich in saponins have long been used in different parts of the world for their detergent properties and they are of great economic interest. Sarasaparilla rich in steroidal saponins is widely used in the manufacture of non-alcoholic drinks. Steroidal saponins are of great pharmaceutical importance as they can be easily transformed into medicinally important steroids such as cortisone, diuretic steroids, vitamin D, sex hormones, and cardiac glycosides. Though total synthesis of therapeutic steroids is today commercially feasible, there is a large demand for plant-derived steroidal sapogenins to be used as starting materials for partial synthesis of these steroids. Diosgenin (Dioscorea sps), hecogenin (Agave sps), sarsasapogenin (Yucca and Smilax sps), and sarmentogenin (Strophanthus sps) are some of the steroidal sapogenins—aglycones of saponins, commercially exploited for semi synthesis of such steroids. Steroidal alkaloids are also commercially employed for steroid manufacture as the semi synthesis involves removal of the ring systems containing the side chain and hence presence of O or N in the side chain is inconsequential.
Because of their surfactant properties, saponins such as quillaic acid (Quillaia bark) are used pharmaceutically for emulsifying fats. Pentacyclic triterpenes are of medicinal importance especially those of the β-amyrin type. Examples include Glycyrhhizin from liquorice, Ginsenosides from Ginseng, Aesculin from Horse chestnut.
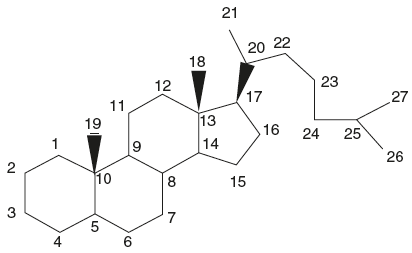
Ring structure of steroidal saponins
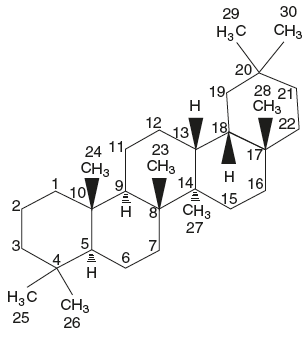
Ring structure of pentacyclic triterpenoid saponins
Leave a Reply