Due to its astringent or protein-precipitating property, tannic acid is used as an antidiarrhoeal and styptic. Formerly it was used in the treatment of burns. It is topically used in the treatment of bed sores, skin ulcerations etc. As an alkaloidal precipitant it is used in alkaloid poisoning. Industrial uses of tannic acid include chemical staining of wood, as a mordant in textile dyeing, iron corrosion proofing and as a clarifying and colour-stabilizing agent in the wine and beer industry.
Description
Tannic acid occurs as yellowish-brown amorphous bulky powder or as flakes or as spongy masses. It has a faint characteristic odour and an astringent taste. It darkens gradually on exposure to air and light. Because of its hygroscopicity it is to be stored in amber-coloured well-closed containers. Commercial tannic acid contains 10% water and does not have a sharp melting point. At 210–215°C it decomposes into pyrogallol and carbon dioxide.
It is soluble in water (1g in 3.5 ml) and glycerol and it is very soluble in alcohol and acetone. Tannic acid is insoluble in benzene, chloroform, ether, petroleum ether, carbon disulphide and carbon tetrachloride.
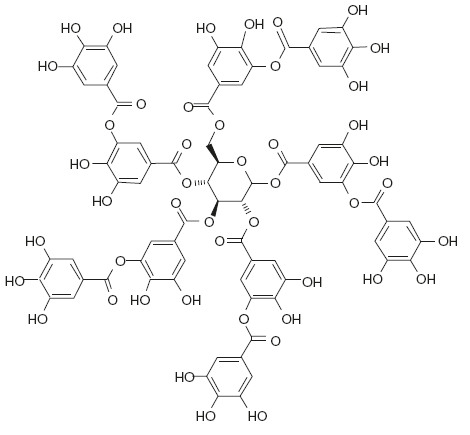
Commercial tannic acid has a molecular formula of C76H52O46. First isolated by Robinson in 1943, tannic acid is not a single homogenous compound, but is a mixture of esters of gallic acid with glucose and its exact composition varies according to its source. For example, tannic acid from Chinese galls (Rhus chinensis) yield on hydrolysis, methyl gallate and 1,2,3,4,5-pentagalloyl glucose. Turkish galls on the other hand yield methyl gallate and a mixture of 1,2,3,6- and 1,3,4,6-tetragalloyl glucose.
Leave a Reply