Chemical change brought about by biological systems such as cells, organelles, enzymes, or cell free extracts resulting in compound conversions or de novo synthesis from added precursors is called bio transformation. Microbial biotransformations have enabled the highly cost-effective manufacture of several medicinally important steroids from plant-derived steroid precursors which are a few steps away from the desired compound.
Similarly, plant cells are able to perform special biotransformation reactions on organic compounds added to the medium. They bring about glycosylation, hydrolysis, esterification, methylation, demethylation, dehydrogenation, epoxidations, and isomerizations, which can even be stereospecific. They can be used to chemically alter natural or synthetic compounds added to the media and thus generate known or newer compounds. Compounds not found in the plant from which the cell culture was made are also formed.
Such selective modifications of pure compounds by plant cells into defined final products are one of the most promising areas of plant cell culture. It’s potential lies in the fact that specific structural modifications on added compounds can be performed much more easily by cultured plant cells than by chemical synthesis or by microbes. There is a huge scope for generation of novel compounds and to transform routine low-value chemicals to high-value pharmaceuticals. There are several successful reports of plant cell biotransformations.
- Cell suspension cultures of Digitalis lanata have effected 12β-hydroxylation of digitoxin (a secondary glycoside of D. purpurea) converting it into digoxin—a therapeutically more useful cardotonic drug. This has resulted in large-scale production of β-methyl digoxin from β-methyl digitoxin in D. lanata cultures. This was a commercially feasible alternative to poor yields seen in normal cultures of D. purpurea and D. lanata. The fact that such cultured cells can efficiently glycosylate, hydroxylate, and acetylate steroid precursors is being commercially exploited in making newer cardenolides from added precursors. Leaf, root, and callus cultures of D. lanata were shown to rapidly transform progesterone to pregnane, thus accumulating a higher quantity of digoxin over normal suspension cultures.
- Vincristine and vinblastine are anti-cancer drugs in high demand, but produced in very poor yields by the source plant Catharanthus roseus. The plant produces vinblastine in higher proportion than vincristine, which is in greater demand due to its better anti-cancer activity. In the plant, these alkaloids are shown to be biogenetically derived by the oxidative coupling of monomeric indoles catharanthine and vindoline (Fig. 1), with vindoline occurring in a higher proportion. Cell culture attempts to generate a higher yield have resulted in the generation of a mixture of monomeric alkaloids with poor yield of coupled useful dimeric alkaloids. This was because of inability of the cultures to produce vindoline, one of the monomers needed for the coupling.
- It can be seen from the figure that peroxidase enzyme is crucial for the coupling of monomers vondoline and catharanthine. Using commercial horse-radish peroxidase enzyme, the cell-free extract of Catharanthus roseus cells containing catharanthine (among other monomers) was made to generate 3, 4-anhydrovinblastine (52.8%) and vinblastine (12.3%) from added vindoline (synthetic or isolated from plants). This possibility was a great leap forward in the production of these anti-cancer alkaloids.
- Today further improved methods of chemical or microbial (N-demethylation) coupling of catharanthine and vindoline as also conversion of vinblastine into more useful vincristine are available. These have therefore contributed to better commercial utilization of cell culture monomers of C. roseus cultures using techniques of biotransformation.
- It is known that the lignan podophyllotoxin is semisynthetically converted to etoposide and teniposide—much valued anti-cancer drugs. Podophyllum peltatum cultures have been found to biotransform precursor synthetic dibenzyl butanolides to lignan derivatives. Similarly, cell suspension cultures of Forsythia intemedia have been shown to effect useful hydroxylation and oxidation reactions, bringing about biotransformation of added podophyllum lignans.
- Arbutin is a skin-whitening agent much prized for use in cosmetic preparations. It has been found that R. serpentina cells in culture accumulate high levels of rauffricine—a glucoalkaloid as against reserpine. When hydroquinone is added as a precursor to the culture medium, the cells glycosylate it and transform it into arbutin and hydroquinone diglycoside at a very high yield (9.2 g/L of arbutin) within 3–4 days of culturing. Several other phenolic compounds are reportedly glycosylated by plant cell cultures.
- Cell cultures of N. tabacum are demonstrated to selectively hydrolyze the R-configurational monoterpenes like bornyl acetate and isobornyl acetate thus bringing about stereospecific conversion into borneol and isoborneol—important aroma chemicals. Likewise, geraniol and nerol are transformed to citral and neral respectively. Several such monoterpene transformations are reported in literature.
- Cell cultures of C. roseus hydroxylated coumarin to form 7-hydroxy coumarin. This in turn was methylated by cell cultures of Ruta graveolens to yield analogs of herniarin, marmesin and other novel compounds.
- Biotransformation reactions mediated by plant cells may be optimized for large-scale production starting with screening for and selecting high-yield strains capable of effecting economically important biotransformations.
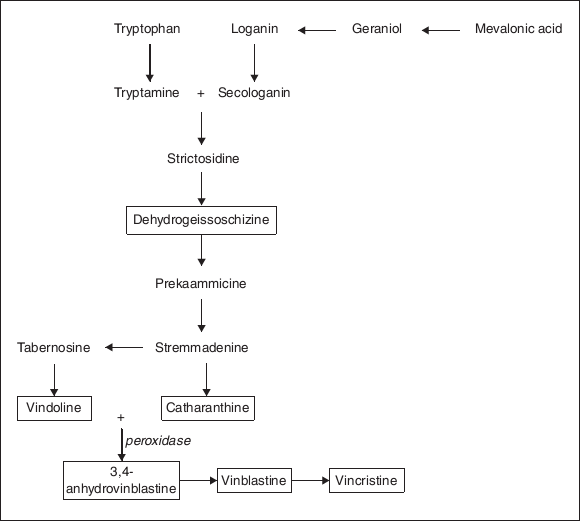
Figure 11.1 Biogenesis of Vincristine and Vinblastine
Leave a Reply