Learning Objective
- Principles involved in the preparation of emulsions
Choice of Emulsion Type
Fats or oils for oral administration are formulated as O/W emulsions. In this form, they are pleasant to take and the inclusion of a suitable flavor in the aqueous phase will mask any unpleasant taste. Emulsions for intravenous administration must also be of O/W type, although intramuscular injections can be formulated as W/O products if a water-soluble drug is required for depot therapy.
Semisolid emulsions for external application can be of O/W or W/O type. O/W type is used for topical application of water-soluble drugs. It is not greasy, is pleasant to use and can be easily washed from skin surfaces. W/O type will act as an occlusive barrier and is useful for cleansing the skin of oil-soluble dirt, but its greasy texture is not acceptable.
Choice of Oil Phase
In many instances, the oil phase of an emulsion is the active agent and its concentration in the product is predetermined. For example, liquid paraffin, castor oil, cod liver oil and arachis oil are medicaments formulated as emulsions for oral administration. Cottonseed oil, soya bean oil and sunflower oil are used in parenteral emulsions for their high calorific value. Turpentine oils and benzyl benzoate are externally applied oils. Many emulsions for external use contain oils that are present as carriers for the active agent.
The type of oil used may also have an effect on the viscosity of the product and the penetration of drug into the skin. For example, in liquid paraffin, hard or soft paraffin can be used individually or in combination with each other to control emulsion consistency. This will ensure that the product spreads easily and forms a thin film over the skin.
Choice of Emulsifying Agent (Emulgent)
Emulsifying agents are the substances added to an emulsion to prevent the coalescence of the globules of the dispersed phase. They are also known as emulgents or emulsifiers. These agents have both a hydrophilic and a lipophilic part in their chemical structure. Emulsifying agents are adsorbed onto the oil–water interface to provide a protective barrier around the dispersed droplets. In addition to this protective barrier, emulsifiers stabilize the emulsion by reducing the interfacial tension of the system. Some emulgents enhance stability by imparting a charge on the droplet surface, thereby reducing the physical contact between the droplets and decreasing the potential for coalescence. Thus, these act in three ways:
- Formation of a protective barrier
- Reduction of interfacial tension
- Decreasing the potential for coalescence by forming an electrical double layer
Emulsifying agents reduce the interfacial tension between the two phases—oil phase and aqueous phase—and thus make them miscible with each other to form a stable emulsion. The choice of the emulgent to be used depends not only on its emulsifying ability but also on its route of administration and its toxicity.
HLB system (Hydrophile–Lipophile balance): Hydrophile–lipophile balance (HLB) method has been devised by Griffin to calculate the relative quantities of the emulgents necessary to produce the most physically stable emulsion or a particular oil–water combination. Each emulsifying agent has a hydrophilic or polar portion and a lipophilic or a nonpolar portion (Fig. 6.3).
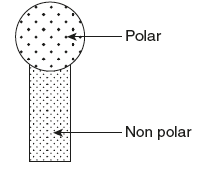
Figure 6.3 Emulsifying Agent
Each portion may be more or less predominant, that is, some agents may have more hydrophilic portion and some may have more lipophilic portion. The HLB system is based on the balance between the hydrophilic and lipophilic portions of the surfactant.
Each surfactant is allocated a HLB number, which represents the relative proportions of its lipophilic and hydrophilic portions. The values are assigned from 1 to 40 and are an indication of the polarity of the substances, but the usual range is between 1 and 20. Emulgents with higher numbers (8–18) indicate hydrophilic properties and produce O/W emulsions, whereas those with lower numbers (3–6) indicate lipophilic properties and produce W/O emulsions.
Table 6.8 shows the classification of agents according to their HLB values and Table 6.9 shows the HLB values of various emulgents.
Table 6.8 Classification of Agents According to Their HLB Values
| Category | HLB Values |
|---|---|
| Antifoaming agents | 1–3 |
| W/O emulsifying agents | 4–6 |
| Wetting agents | 7–9 |
| O/W emulsifying agents | 8–18 |
| Solubilizers | 15–20 |
| Detergents | 13–15 |
Table 6.9 HLB Values of Various Emulgents
| Emulgents | HLB Values |
|---|---|
| Oleic acid | 4.3 |
| Sorbitan monolaurate (Span 20) | 8.6 |
| Sorbitan monostearate (Span 60) | 4.7 |
| Sorbitan monooleate (Span 80) | 4.3 |
| Sorbitan trioleate (Span 85) | 1.8 |
| Polysorbate 20 (Tween 20) | 16.7 |
| Polysorbate 60 (Tween 60) | 14.9 |
| Polysorbate 80 (Tween 80) | 15.0 |
| Potassium oleate | 20.0 |
| SLS | 40.0 |
| Acacia | 8.0 |
| Tragacanth | 13.2 |
Leave a Reply