Learning Objective
- Different classes of emulsifying agents used to stabilize an emulsion
There are two types of emulsifying agents (Figure 6.4):
- Primary (true) emulsifying agents, which produce emulsions with good stability
- Secondary emulsifying agents or emulsion stabilizers, which are used in combination with primary emulsifying agents to improve the stability of the emulsion
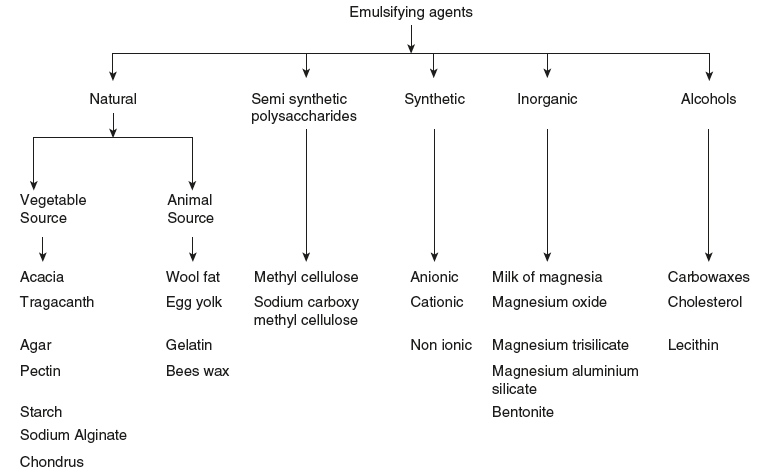
Figure 6.4 Classification of Emulsifying Agents
The emulsifying agents are classified into the following categories:
- Natural products: The following are the different natural emulsifying agents:
- Acacia is the best emulsifying agent for the extemporaneous preparation of emulsions. Preparations of good quality, stability and appearance can be made only with a mortar and pestle. This is because the concentrated emulsion produced in the initial stage of preparation is very viscous and sticky. Therefore, the oil cannot escape the vigorous shearing action of the pestle and is easily reduced to fine globules.Acacia emulsions are of low viscosity. Therefore, thickening agents such as tragacanth and sodium alginate are added. These emulsions are palatable and are stable over a wide pH range (2–10).
- Tragacanth is rarely used alone because of its high viscosity. The emulsions are coarse and are used as a stabilizer in acacia emulsions in the proportion 1:10 (acacia).
- Sodium alginate has high viscosity and is used as an emulsion stabilizer along with acacia.
- Agar is the dried extract obtained from certain seaweeds. It is used as an emulsion stabilizer in liquid paraffin emulsions prepared with acacia. It is soluble in boiling water, producing solutions of high viscosity.
- Starch is a poor emulsifying agent and is used for the preparation of enemas containing oils.
- Pectin is obtained from the inner rind of citrus fruits or from the apple pulp that remains after the making of cider. In acid media, it is a good O/W emulgent but degrades in alkaline pH. It is employed in the preparation or stabilizing of cosmetic creams and lotions.
- Chondrus is dried seaweed. It is not suitable for small-scale emulsification because preparation of the mucilage is time consuming and its emulsions must be homogenized. It is used in the emulsification of cod liver oil emulsions to mask the unpleasant odor and taste of the oil; 2.5% mucilage will emulsify an equal volume of fixed oil.
- Wool fat is a type of wax that consists chiefly of fatty acid esters of cholesterol and other sterols together with normal fatty alcohols. It can absorb 50% of water, but when mixed with other fatty substances it can emulsify several times its own weight of aqueous or hydroalcoholic liquids. The emulsions made are of W/O type.
- Gelatin is used for the emulsification of liquid paraffin emulsions at a concentration of 1%. Gelatin emulsions are prone to bacterial growth, and therefore, a suitable preservative should be added.
- Egg yolk is an emulsifying agent because of the presence of lecithin and cholesterol. It is rarely used in industrial preparations as they are spoiled during transportation or if not preserved properly. It is used for the emulsification of fish liver oils.
- Semisynthetic polysaccharides: The following are the different semisynthetic polysaccharides:
- Methyl cellulose is of low viscosity and is suitable as emulgents and emulsion stabilizers. It is suitable for emulsifying mineral and vegetable oils and is used at a concentration of 2%.
- SCMC is of medium viscosity grade and is used at a concentration of 0.5% to 1% as emulsion stabilizer.
- Synthetic polysaccharides: These are further classified into the following types:
- Anionic: In aqueous solution, these substances ionize into a large anion, which is responsible for their emulsifying ability, and a small cation. These are of five types and bear a negative charge on them.
- Alkali metal and ammonium soaps
- Soaps of divalent and trivalent metals
- Amine soaps
- Alkyl sulfates
- Alkyl phosphates
- Alkali soap emulsions (monovalent soaps) are stable above pH 10 but are sensitive to acids. High concentration of electrolytes can salt out the soap. They are incompatible with polyvalent cations (Mg2+, Al3+, Zn2+), as they can cause phase reversal. Their physiological action and unpleasant taste make them unsuitable for internal emulsions. Moreover, because of their alkaline pH, they should not be used in preparations for application to broken skin. They can be used as emulgents only in O/W emulsions.(Note: Alkali metal and ammonium soaps are the sodium, potassium or ammonium salts of long chain fatty acids such as oleic, stearic and ricinoleic acids. They produce O/W emulsions that can be prepared with either of the following:
- A preformed soap such as soft soap (turpentine liniment)
- A soap formed during the preparation of emulsion such as ammonium soap made from oleic acid and ammonia (white liniment)
- Among the soaps of divalent and trivalent metals, usually only the calcium soaps are used as W/O emulsifying agents. They cannot be used internally, but they are less alkaline and less sensitive to acid.(Note: Ca2+, Mg2+, Al3+ and Zn2+ salts of fatty acids are also W/O emulsifying agents, but only calcium soaps are commonly used.)
- Triethanolamine (amine) soaps produce fine-grained, almost neutral (pH 7.5 to 8) O/W emulsions. They can be applied to broken skin but are unsuitable for internal use.
- Alkyl sulfates are esters of fatty alcohols and sulfuric acid. Among alkyl sulfates, SLS is used alone to produce O/W emulsions of low stability. However, when it is used in conjunction with fatty alcohols, products of excellent stability and quality are obtained.
- Alkyl phosphates are also used in combination with fatty alcohols. They are similar to alkyl sulfate but the alcohols are phosphated instead of sulfated.
- Cationic: In aqueous solution, these substances ionize into a large cation, which is responsible for their emulsifying ability, and a small anion. They bear a positive charge on them, for example, quaternary ammonium compounds. They have emulgent properties apart from their disinfectant and preservative properties. If used alone, their emulsifying power is poor, but they produce emulsions of great stability when combined with fatty alcohols. Examples are benzalkonium chloride, benzethonium chloride and cetrimide (cetyl trimethyl ammonium bromide).
- Nonionic: They do not ionize in aqueous solutions. The emulsion prepared is stable over a wide range of pH and is not affected by the addition of acids and electrolytes. Examples are glycol and glycerol esters (glyceryl monostearate), sorbitan esters (spans), polysorbates (tweens), macrogols (polyethylene glycol) and polyvinyl alcohol.
- Anionic: In aqueous solution, these substances ionize into a large anion, which is responsible for their emulsifying ability, and a small cation. These are of five types and bear a negative charge on them.
- Inorganic agents: Finely divided solids with suitable balanced hydrophobic and hydrophilic properties are adsorbed at an oil–water interface, forming a coherent film that prevents coalescence of the dispersed globules. If the solid particles are preferentially wetted by the oil, then W/O emulsions are formed, whereas wetting by water results in O/W products. Examples are milk of magnesia (10%–20%), magnesium oxide (5–10%) and magnesium aluminum silicate (1%).
- Alcohols: These are further classified into the following types:
- Carbowaxes are used in the preparation of ointments and creams. The substances with a molecular weight of 200–700 are viscous, light-colored, hygroscopic liquids, whereas those with a molecular weight above 1000 are wax-like solids.
- Cholesterol is used only in combination with other emulsifying agents to produce a stable emulsion.
- Lecithin
Leave a Reply