Creaming is the separation of an emulsion into two regions, one of which is richer in the disperse phase than the other. It is not a serious instability problem as a uniform dispersion can be reobtained by shaking the emulsion. However, there may be coalescence of droplets as they are present close to each other. Larger droplets may cream more rapidly and coalesce more readily in the cream layer; therefore, the emulsion may eventually crack. It is a temporary or reversible process.
If the disperse phase is less dense than the continuous phase, as in O/W emulsions, the velocity of sedimentation becomes negative and upward creaming results.
If the internal or disperse phase is heavier than the external phase, the globules settle, as in W/O emulsions, where the internal aqueous phase is denser than the continuous oil phase. This effect is known as downward creaming.
Stoke’s law is used to express the velocity of sedimentation.
According to Stoke’s law 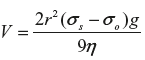 or
or 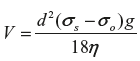
where V = velocity in cm/s (rate of creaming)
r = radius of the globules
d = diameter of the globules in cm
σs = density of dispersed phase
σo = density of dispersion medium
g = gravitational constant
η = viscosity of the dispersion medium
The factors in Stoke’s equation can be altered to reduce the rate of creaming in an emulsion.
- By decreasing the diameter of the oil globules, the rate of creaming decreases. This is because small globules cream less rapidly than large ones. This can be achieved by the use of a homogenizer.
- The viscosity of the external or continuous phase can be increased by adding a thickening agent. Increased viscosity will retard the movement of globules. The inclusion of methyl cellulose will reduce the mobility of the dispersed droplets in an O/W emulsion. The addition of soft paraffin will have the same effect on water droplets in a W/O emulsion.
- The possibility of creaming is decreased by the reduction in density differences between the two phases. Creaming can be prevented if the densities of the two phases are identical.
- Storage in a cool place can decrease creaming. Temperature rise decreases the viscosity of the continuous phase and increases the number of collisions between the globules. Freezing of the aqueous phase must be avoided since ice may separate and cause cracking by exerting pressure on the globules.
Leave a Reply