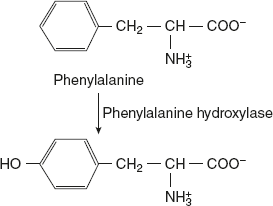
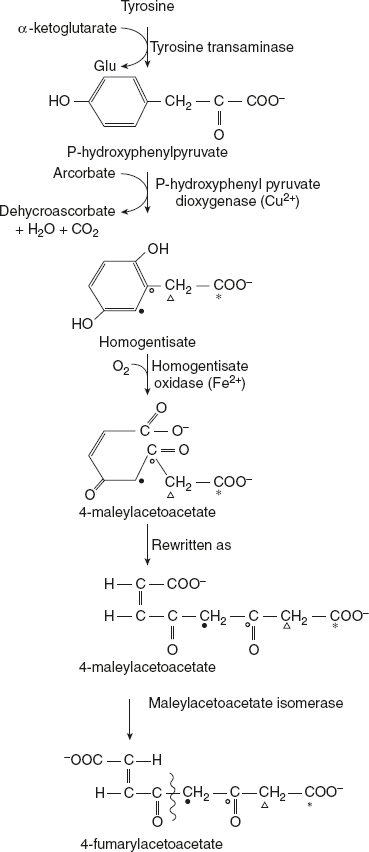
As phenylalanine is converted to tyrosine, a single pathway is responsible for the degradation of both these amino acids, which occurs mostly in liver. In a sequence of reactions, tyrosine is converted to fumarate and acetoacetate. Tyrosine first undergoes transamination to 5-p-hydroxyphenyl pyruvate. P-hydroxyphenyl pyruvate dioxygenase is a copper-containing enzyme. It catalyses oxidative decarboxylation as well as hydroxylation of the phenyl ring of p-hydroxyl-phenyl pyruvate to produce homogentisate. This reaction involves a shift in hydroxyl group at para position to meta position and incorporates a new hydroxyl group at para position. This step in tyrosine metabolism requires ascorbic acid.
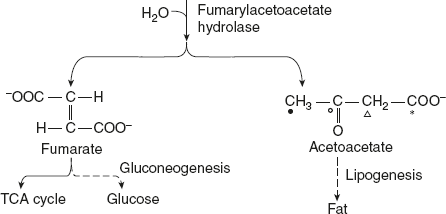
Homogentisate oxidase cleaves the benzene ring of homogentisate to form 4-male-ylacetoacetate. Molecular oxygen is required for this reaction to break the aromatic ring. Maleylacetoacetate undergoes isomerisation to form 4-fumarylacetoacetate, and this reaction is catalysed by maleylacetoacetate isomerase. Fumarylacetoacetase brings about the hydrolysis of fumarylacetoacetate to liberate fumarate and acetoacetate.
Fumarate is an intermediate of citric acid cycle and also serves as precursor for gluconeogenesis. Acetoacetate is a ketone body, from which fat can be synthesised.
Leave a Reply