- Most of the amino acids are soluble in water and insoluble in organic solvents.
- Melting points: Amino acids generally melt at higher temperature, and it is above 200°C.
- Taste: Amino acids may be sweet, tasteless, and bitter.Sweet – Gly, Ala, and ValTasteless – LeuBitter – Arg and IleSodium glutamate is employed as a flavouring agent in food industry to increase taste and flavour.
- Optical properties: All amino acids, except glycine, possess optical isomers due to the presence of asymmetric α-carbon atom.
- Ampholytes: Amino acids contain both acidic (COOH) and basic (NH2) groups. They can donate a proton or accept a proton. Hence, amino acids are regarded as ampholytes.
- Zwitterion or dipolar ion: The name zwitter means hybrid. Zwitterion (or dipolar ion) is a hybrid molecule containing positive and negative ionic groups. The amino acids rarely exist in a neutral form with free carboxylic (—COOH) and free amino acid (—NH2) groups.
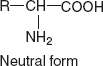 In a strongly acidic pH (< 7 pH) – low pH, the amino acid is positively charged (cation), while in a strongly alkaline pH (>7 pH) – high pH, it is negatively charged (anion). Each amino acid has a characteristic pH (e.g. Leu pH is 6), at which it carries both positive and negative charges and exists as zwitterion.
In a strongly acidic pH (< 7 pH) – low pH, the amino acid is positively charged (cation), while in a strongly alkaline pH (>7 pH) – high pH, it is negatively charged (anion). Each amino acid has a characteristic pH (e.g. Leu pH is 6), at which it carries both positive and negative charges and exists as zwitterion.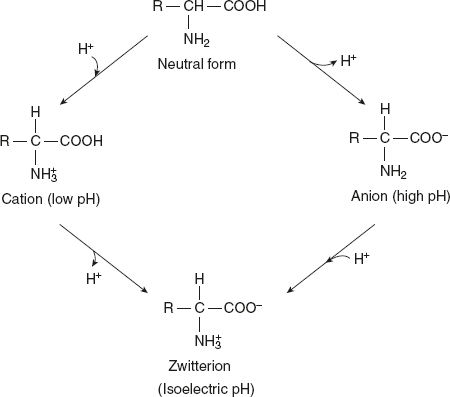
 In a strongly acidic pH (< 7 pH) – low pH, the amino acid is positively charged (cation), while in a strongly alkaline pH (>7 pH) – high pH, it is negatively charged (anion). Each amino acid has a characteristic pH (e.g. Leu pH is 6), at which it carries both positive and negative charges and exists as zwitterion.
In a strongly acidic pH (< 7 pH) – low pH, the amino acid is positively charged (cation), while in a strongly alkaline pH (>7 pH) – high pH, it is negatively charged (anion). Each amino acid has a characteristic pH (e.g. Leu pH is 6), at which it carries both positive and negative charges and exists as zwitterion.
Leave a Reply