The existence of different ionic forms of amino acids can be more easily understood by the titration curves as shown in Figure 3.1. At low pH, leucine exists in a fully protonated form as cation. As titration proceeds with NaOH, leucine loses its proton, and at isoelectric pH (pI), it blames a zwitterion. Further titration results in the formation of anionic form of leucine. Further titration results in the formation of anionic forms of leucine as shown in Figure 3.1.
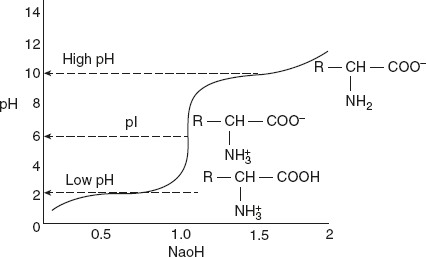
Figure 3.1 Titration Curve of Amino Acid Leucine
Leave a Reply