Alkali metal cations bind only weakly to form complexes with enzymes, but K+, the most abundant intracellular cation, is known to activate a great many enzymes, particularly those catalysing phosphoryl transfer or elimination reaction. It appears that K− is largely bound to be a negatively charged group on an inactive form of the enzyme and thus causes a change in conformation to a more active form.
Example: Muscle pyruvate kinase – tetrameric enzyme
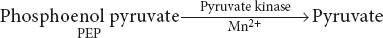
This reaction is required for alkali metal cations and Mn2+ (or Mg2+), all of which bind in the region of the active site. The carboxyl group of PEP binds to the enzyme bound K+, whereupon a conformational change takes place which facilitates the progress of the reaction via an E-Mn2+-PEP complex.
Leave a Reply