Cu, Zn, Mo, Fe, and Co cations. Transition metal ions bind to enzymes much more strongly than the metal ions and form metalloenzymes. Mo and Fe are found in nitricoxide reductase; Fe is a component of Hb, the ![]() carrying haemoprotein of erythrocytes. Co is found in vitamin B12. Superoxide dismutase is a copper-metalloenzyme, which catalyses the removal of highly reactive O2-produced.
carrying haemoprotein of erythrocytes. Co is found in vitamin B12. Superoxide dismutase is a copper-metalloenzyme, which catalyses the removal of highly reactive O2-produced.
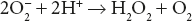
Bovine erythrocyte superoxide dismutase is a dimeric protein containing two Cu2+ ions and two Zn2+ ions. The Zn2+ ions appear to have a structural rather than a catalytic role, while Cu2+ ions are involved in reaction sequence.
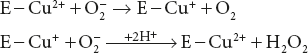
In contrast to above, Zn2+ has a catalytic role in the reaction catalysed by carboxypeptidase A, where the C-terminal amino acid of a polypeptides is removed, provided it has a non-polar side chain.
Carboxypeptidase A – Zn2+ C-terminal acid of polypeptide is removed. Bovine pancreas carboxypeptidase A is a monomeric enzyme which contains one atom of Zn. The active site contains the coordinated Zn2+ ions bound to histidine-69, glumate-72, histidine-196, and H2O as well as a groove for the polypeptide substrate and hydrophobic pocket for binding the side chain of the C-terminal amino acid. The terminal carboxyl group of the substrate forms an electrostatic interaction with arginine-145 (Figure 6.36).
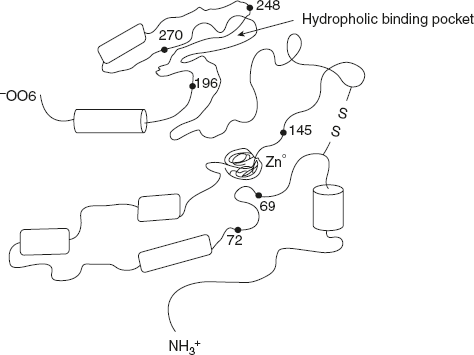
Figure 6.36 A Simplified Representation of the Three-dimensional Structure of Carboxypeptidase A
Tyrosine-248 is located in such a position in the enzyme-substrate complex that it could donate a proton to the nitrogen of the peptide bond being hydrolysed, and a carboxyl group of glutamate-270 acts as a general base catalyst to make the attacking water molecule nucleophilic.
The amino acids Arginine in position 145, glutamate in 270, and tyrosine-248 are moving to the substrate and forcing water molecules out of active site, thereby creating a hydrophobic environment for the substrate to bind.
Leave a Reply