Reducing Properties
Sugars that are oxidised at C-1 are known as reducing sugars. Sugars can react with the chemical agents due to the presence of free carbonyl (aldehyde or ketone) groups. In the alkaline medium, they form enediols, which reduce Cu2+, Ag+, Fe3+, and Bi+3 and oxidise to the corresponding sugar acids. Such sugars are known as reducing sugars, for example, glucose and fructose. The reducing property of sugars is used for the quantitative and qualitative determination of sugars. Some of the qualitative tests are explained here:
Barfoed’s test:Barfoed’s reagents contain a solution of copper acetate in acetic acid. Monosaccharides react with Barfoed’s reagent and give a red precipitate. Monosaccharides react faster than disaccharides and this test helps to differentiate between them.
Benedict’s test:Benedict’s reagents contain Cu2+ (cupric sulphate), alkali (sodium carbonate), and sodium citrate. The role of sodium citrate is to prevent the precipitation of copper hydroxide or carbonate by forming complexes with Cu2+ ions. On heating the sugar with Benedict’s reagent, the reducing sugar forms enediol in an alkaline medium, which reduces Cu2+ to Cu+. This forms a red-coloured precipitate. In Fehling’s solution, potassium tartrate is used instead of sodium citrate and KOH is the alkaline medium.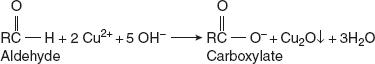
Leave a Reply