- HydrolysisFats, being esters of glycerol and fatty acids, are readily hydrolysed to glycerol and fatty acids by acids, alkalis, superheated steam, and enzymes known as lipases, which act on the ester linkages. Two kinds of hydrolysis are known as enzyme hydrolysis and alkali hydrolysis.Enzyme hydrolysis is affected by lipase of pancreatic juice. This is important in the digestion of fats. The process of alkali hydrolysis is called saponification, which means ‘soap making’. The alkali salt resulting from saponification is soap.
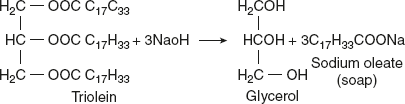 SoapsThe common fats used for making soaps contain predominantly palmitic, stearic, and oleic acids. The soaps we use for washing consist largely of the sodium or potassium soaps of these fatty acids. The potassium soaps are soft and soluble, and the sodium soaps are hard and less soluble in water. When soluble soap is used in hard water, which contains calcium and magnesium ions, the efficiency of producing lather is diminished. This is because the fatty acids in the soap are precipitated by the calcium and magnesium salts of the hard water. The precipitation continues until all the calcium and magnesium ions combine with the fatty acids of the soap.Soaps are good cleansing agent because they are effective emulsifying agents. Dirt is held to the surface by greasy substances. Soaps, being good emulsifying agents, lower the surface tension also. The greasy materials are emulsified by the soaps, and dirt is carried away with them in washing.Reactions of soap in dilute solution: A strong solution of pure soap in water is clear whereas, when this is diluted with more water, the solution becomes cloudy and opalescent. This is because on dilution, soaps undergo partial hydrolysis with liberation of insoluble fatty acids, which make the solution cloudy. RCOONa + H2O
SoapsThe common fats used for making soaps contain predominantly palmitic, stearic, and oleic acids. The soaps we use for washing consist largely of the sodium or potassium soaps of these fatty acids. The potassium soaps are soft and soluble, and the sodium soaps are hard and less soluble in water. When soluble soap is used in hard water, which contains calcium and magnesium ions, the efficiency of producing lather is diminished. This is because the fatty acids in the soap are precipitated by the calcium and magnesium salts of the hard water. The precipitation continues until all the calcium and magnesium ions combine with the fatty acids of the soap.Soaps are good cleansing agent because they are effective emulsifying agents. Dirt is held to the surface by greasy substances. Soaps, being good emulsifying agents, lower the surface tension also. The greasy materials are emulsified by the soaps, and dirt is carried away with them in washing.Reactions of soap in dilute solution: A strong solution of pure soap in water is clear whereas, when this is diluted with more water, the solution becomes cloudy and opalescent. This is because on dilution, soaps undergo partial hydrolysis with liberation of insoluble fatty acids, which make the solution cloudy. RCOONa + H2O  (RCOOH + NaOH)The liberation of NaOH in this way accounts for the alkalinity of ordinary washing soap, which, if used on the skin, has a deleterious effect. This disadvantage is overcome in the preparation of toilet soaps by the addition of excess of fatty acids or by using large proportion of sodium oleate, which is not so readily hydrolysed in water.
(RCOOH + NaOH)The liberation of NaOH in this way accounts for the alkalinity of ordinary washing soap, which, if used on the skin, has a deleterious effect. This disadvantage is overcome in the preparation of toilet soaps by the addition of excess of fatty acids or by using large proportion of sodium oleate, which is not so readily hydrolysed in water.
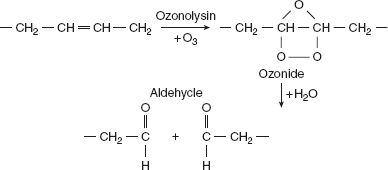
- AutoxidationUnsaturated fatty acids in fats react with ozone and oxygen to undergo a reaction called autoxidation, which is the presence of appropriate amount of moisture, light, warmth, and catalytic substances. Ozonides, peroxides, aldehyde, and ketones are formed.
- With ozone, an unstable ozonides is formed, which later cleaves by water to give rise to two aldehyde groups.
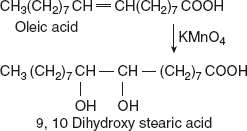
- With KMnO4, under mild condition, the glycols are formed at the sites of double bonds.
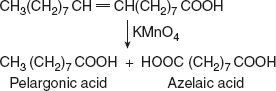
- Under vigorous conditions, the same reagent cleaves the molecule at the double bond and oxidises the terminal portions to the carboxyl group.
 SoapsThe common fats used for making soaps contain predominantly palmitic, stearic, and oleic acids. The soaps we use for washing consist largely of the sodium or potassium soaps of these fatty acids. The potassium soaps are soft and soluble, and the sodium soaps are hard and less soluble in water. When soluble soap is used in hard water, which contains calcium and magnesium ions, the efficiency of producing lather is diminished. This is because the fatty acids in the soap are precipitated by the calcium and magnesium salts of the hard water. The precipitation continues until all the calcium and magnesium ions combine with the fatty acids of the soap.Soaps are good cleansing agent because they are effective emulsifying agents. Dirt is held to the surface by greasy substances. Soaps, being good emulsifying agents, lower the surface tension also. The greasy materials are emulsified by the soaps, and dirt is carried away with them in washing.Reactions of soap in dilute solution: A strong solution of pure soap in water is clear whereas, when this is diluted with more water, the solution becomes cloudy and opalescent. This is because on dilution, soaps undergo partial hydrolysis with liberation of insoluble fatty acids, which make the solution cloudy. RCOONa + H2O
SoapsThe common fats used for making soaps contain predominantly palmitic, stearic, and oleic acids. The soaps we use for washing consist largely of the sodium or potassium soaps of these fatty acids. The potassium soaps are soft and soluble, and the sodium soaps are hard and less soluble in water. When soluble soap is used in hard water, which contains calcium and magnesium ions, the efficiency of producing lather is diminished. This is because the fatty acids in the soap are precipitated by the calcium and magnesium salts of the hard water. The precipitation continues until all the calcium and magnesium ions combine with the fatty acids of the soap.Soaps are good cleansing agent because they are effective emulsifying agents. Dirt is held to the surface by greasy substances. Soaps, being good emulsifying agents, lower the surface tension also. The greasy materials are emulsified by the soaps, and dirt is carried away with them in washing.Reactions of soap in dilute solution: A strong solution of pure soap in water is clear whereas, when this is diluted with more water, the solution becomes cloudy and opalescent. This is because on dilution, soaps undergo partial hydrolysis with liberation of insoluble fatty acids, which make the solution cloudy. RCOONa + H2O  (RCOOH + NaOH)The liberation of NaOH in this way accounts for the alkalinity of ordinary washing soap, which, if used on the skin, has a deleterious effect. This disadvantage is overcome in the preparation of toilet soaps by the addition of excess of fatty acids or by using large proportion of sodium oleate, which is not so readily hydrolysed in water.
(RCOOH + NaOH)The liberation of NaOH in this way accounts for the alkalinity of ordinary washing soap, which, if used on the skin, has a deleterious effect. This disadvantage is overcome in the preparation of toilet soaps by the addition of excess of fatty acids or by using large proportion of sodium oleate, which is not so readily hydrolysed in water.


Leave a Reply