Complex II is called succinate dehydrogenase complex. It is the only membrane-bound enzyme in TCA. Complex II is smaller and simpler than complex I. However, it contains two types of prosthetic groups and at least four different proteins. To the first protein FAD is covalently bound and Fe-S centre with four Fe atoms. In addition, a second protein is present. Electrons pass form succinate to FAD and then through Fe-S centres to ubiquinone. Other substrates for mitochondrial dehydrogenase pass electrons into the respiratory chain at the level of ubiquinone but not through the complex II.
First step in β-oxidation of fatty acyl-CoA catalysed by flavoprotein acyl-CoA dehydrogenase involves transfer of electrons form the substrate to the FAD of the dehydrogenase and then to electron-transferring flavoprotein (ETF), which in turn passes its electrons to ETP, ubiquinone oxidoreductase. This enzyme passes electrons into the respiratory chain by reducing ubiquinone.
Glycerol-3-phosphate, formed either from glycerol released by triacylglycerol breakdown or by the reduction of dihydroxyacetone phosphate from glycolysis is oxidised by glycerol-3-phosphate dehydrogenase (Refer Figure 8.17). This enzyme is a flavoprotein, located on the outer face of the inner mitochondrial membrane, and it channels the electrons in respiratory chain by reducing ubiquinone.
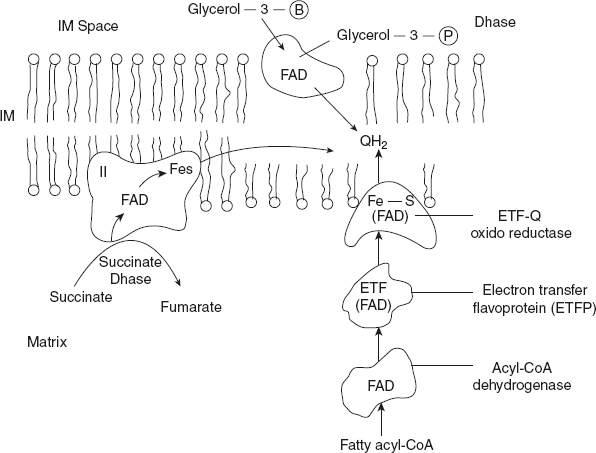
Figure 8.17 Pathway of Electrons Flow from NADH, Succinate, Fatty acyl-CoA, Glycerol-3-phosphate to Ubiquinone
Leave a Reply