The pyrimidine nucleotides undergo similar reaction (dephosphorylation, deamination, and cleavage of glycosidic bond) like that of purine nucleotides to liberate the nitrogenous bases cytosine, uracil, and thymine. The bases are then degraded to highly soluble products β-alanine and β-aminoisobutyrate and other reactions to finally produce acetyl CoA and succinyl CoA. The degradation of pyrimidine nucleotides is represented in Figure 11.9.
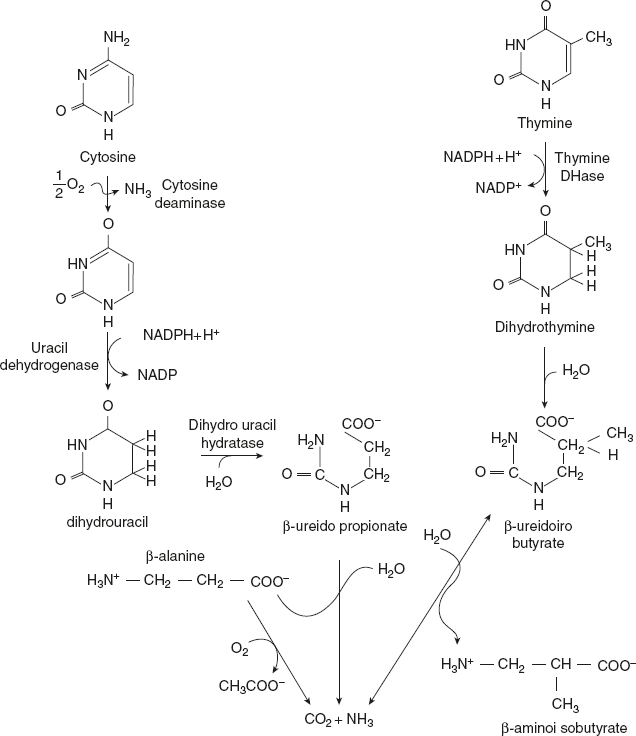
Figure 11.9 Degradation of Pyrimidine Nucleotides
Leave a Reply