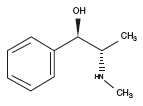
Ephedrine occurs as a waxy solid or as crystalline particles. Its IUPAC name is (R*,S*)-2-(methylamino)-1-phenylpropan-1-ol and has the molecular formula C10H15NO1. It has a bitter taste, is odourless or has a slight aromatic odour. In warm weather it slowly volatilizes. The anhydrous substance melts at 36°C and the hemi-hydrate melts at 42°C. It is a weak base, with a pKa of 9.6. Ephedrine decomposes with light. Solutions in oil can have a garlicky odour. It is soluble in water (1 in 20) and in alcohol, chloroform, ether, glycerol, olive oil and in liquid paraffin (Windholz, 1983).
Ephedrine and its optical isomer pseudoephedrine are structurally very similar to methamphetamine. In illicit drug laboratories simple dehydrogenation is used to make methamphetamine from ephedrine. Ephedrine exhibits optical isomerism and has two chiral centres, giving rise to four stereoisomers. By convention the pair of enantiomers with the stereochemistry (1R,2S and 1S,2R) is designated ephedrine, while the pair of enantiomers with the stereochemistry (1R,2R and 1S,2S) is called pseudoephedrine.
Ephedrine is a substituted amphetamine and a structural methamphetamine analogue. It differs from methamphetamine only by the presence of a hydroxyl (OH). Amphetamines, however, are more potent and have additional biological effects.
The isomer which is marketed is (–)-(1R,2S)-ephedrine. Ephedrine hydrochloride is a white crystalline powder. Most of the L-ephedrine produced today for official medical use is made synthetically as the extraction and isolation process from the plant source is tedious and no longer cost effective.
Isolation
- The plant material is ground to a fine powder, passed through #40 mesh and percolated with 80% alcohol until the percolate is colourless.
- The percolate is concentrated to a syrupy fluid on a rotary vacuum evaporator. It is diluted with equal volume of water, rendered alkaline with ammonium hydroxide and filtered.
- Extract the filtrate with chloroform and heat the residue on the filter under reflux with chloroform.
- Combine the chloroform extracts, concentrate to a small volume and set aside overnight for spontaneous evaporation.
- A greenish fragrant gelatinous residue that is formed is dissolved in a small volume of hot water and treated with dilute hydrochloric acid until exactly neutral to litmus.
- The solution is carefully evaporated to dryness and the residual alkaloidal salt is purified by recrystallization from absolute alcohol.
- Base ephedrine is regenerated from a solution of the alkaloidal salt by first alkalinizing with ammonium hydroxide.
- It is then extracted with chloroform, which is further evaporated to yield white rosette crystals of ephedrine that are easily broken up into coarse needles.
Leave a Reply