Learning Objective
- Basic principle and different types of emulsions
Emulsions are biphasic heterogeneous systems consisting of two immiscible phases, one of which (the dispersed phase) is finely subdivided and uniformly distributed as droplets throughout the other (the dispersion medium). An emulsion is rendered homogeneous by the addition of an emulsifying agent. The emulsifying agent ensures that the droplets (dispersed phase) is finely dispersed throughout the dispersion medium as minute globules.
There are two types of emulsion:
- Oil in water (O/W) emulsions: The oil (internal or dispersed) phase is dispersed as droplets throughout the aqueous phase (external or continuous phase or dispersion medium). Oil is the dispersed phase and water is the dispersion medium.
- Water in oil (W/O) emulsions: The internal phase is composed of water droplets and the external phase is nonaqueous. Water is the dispersed phase and oil is the dispersion medium.
In general, all oral emulsions tend to be oil-in-water as the oily phase is usually less pleasant to take and more difficult to flavor. The differences between O/W and W/O emulsions are provided in Table 6.6.
The major use of emulsions is as cream formulations (for external application). However, they may also be administered intravenously, rectally or orally. Emulsions are physically unstable and the various excipients in the formulation are present primarily to stabilize the physical properties of the system.
Table 6.6 Differences between O/W and W/O Emulsions
| Si.No. | Oil in Water Emulsion (O/W) | Water in Oil Emulsion (W/O) |
|---|---|---|
| 1. | Oil is the dispersed phase and water is the dispersion medium. | Water is the dispersed phase and oil is the dispersion medium. |
| 2. | They are nongreasy and easily washed from the skin surface. | They are greasy and not easily washed by water. |
| 3. | They are used externally to provide a cooling effect, for example, vanishing cream. | They are used externally to prevent evaporation of moisture from the surface of skin, for example, cold cream. |
| 4. | Water-soluble drugs are more quickly released from O/W emulsions. | Oil-soluble drugs are more quickly released from W/O emulsions. |
| 5. | They are preferred for oral formulations as the taste of oils can be masked. | They are preferred for topical preparations such as creams. |
| 6. | O/W emulsions give a positive conductivity test since the external phase is water, which is a good conductor of electricity. | W/O emulsions do not give a positive conductivity test since the external phase is oil, which is a poor conductor of electricity. |
| 7. | 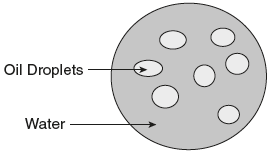 | 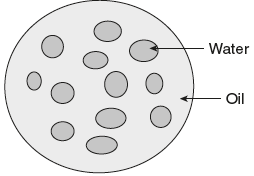 |
Leave a Reply