This theory was proposed by a German biochemist Emil Fischer in 1898. This is, in fact, the very first model proposed to explain an enzyme-catalysed reaction. According to this model, the substrate or conformation of enzyme is rigid. The substrate fits to the binding site (active site) just as a key fits to the proper lock or a hand into the proper glove as shown in Figure 6.15. Thus, the active site of an enzyme is a rigid and pre-shaped template where only a specific substrate can bind.
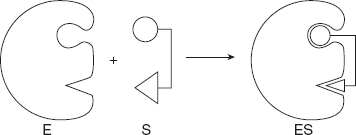
Figure 6.15 Diagrammatic Representation of Lock and Key Model
In fact, the enzyme and substrate union depend on molecular substrate of enzyme and substrate. This is also known as concept of intermolecular fit. This enzyme substrate complex is highly unstable and decomposes to produce free enzyme. The ES complex union results in the release of energy. It is this energy, which, in fact, raises the energy level of the substrate molecule, thus, inducing the activated state. In this activated stage, certain bonds of substrate molecule become more susceptible to cleavage.
This model does not give any scope for flexible nature of enzymes. Here, the model totally fails to explain many facts of enzymatic reactions, the most important being the effect of modulators.
Leave a Reply