Learning Objectives
- Introduction to gels and the types of gels
- Discussion on gelling agents
- Evaluation of gels
Gels are semisolid preparations usually homogeneous and clear, consisting of a liquid phase within a three-dimensional polymeric matrix that is physically or sometimes chemically cross-linked by suitable gelling agents. Gels are applied to the skin or certain mucous membranes for various purposes such as protective, therapeutic or prophylaxis.
Alternatively, it is a coherent system in which a liquid phase is entrapped within a polymeric matrix (natural or synthetic gum) of high degree physical or chemical cross-linking. Gels are transparent or translucent, nongreasy semisolid preparations. Some are as transparent as water, in an aesthetically pleasing state, whereas others are turbid as the polymer is present in colloidal aggregates that disperse light. They are mainly used for medication or lubrication.
Hydrophobic gels: Hydrophobic gel (oleogel) bases usually consist of liquid paraffin with polyethylene or fatty oils gelled with colloidal silica or aluminum or zinc soaps.
Hydrophilic gels: Hydrophilic gel (hydrogel) bases usually consist of water, glycerol, or propylene glycol gelled with suitable agents such as tragacanth, starch, cellulose derivatives, carboxyvinyl polymers, and magnesium aluminum silicates.
Properties of Gels
- Thixotropy: Gels become fluid when agitated, but resolidify on resting. In general, gels are apparently solid, jellylike materials. By replacing the liquid with gas, it is possible to prepare aerogels, possessing exceptional properties such as very low density, high specific surface area, and excellent thermal insulation properties.
- Syneresis: On standing for some time, the gel often shrinks because some of the liquid is squeezed out of the system.
- Swelling: This is exactly opposite to syneresis, wherein the gel takes up some liquid and increases in volume.
- Imbibition: The gel takes up a certain amount of liquid with no considerable increase in volume.
Common gelling agents used are polyvinyl alcohol, sodium polyacrylate, acrylate polymers, and copolymers with an abundance of hydrophilic groups.
Types of Gels
Figure 7.2 shows the classification of gels.
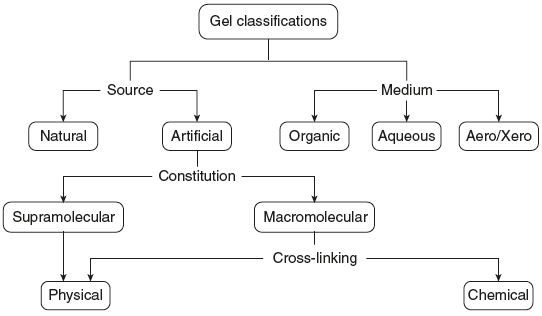
Figure 7.2 Classification of Gels
Leave a Reply