Koshland (1958) proposed a more susceptible and realistic model for enzyme-substrate complex formation. As per this model, the active site is rigid or pre-shaped. The essential features of the substrate binding site are present at the nascent active site. The interaction of the substrate with the enzyme induces fit or a conformation change in enzyme, resulting in the formation of a strong binding site as shown in Figure 6.16. Further, due to induced fit, the appropriate amino acids of the enzyme are reciprocal to form the active site and bring about the catalysis.
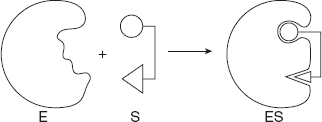
Figure 6.16 Diagrammatic Representation of Induced Fit Model
To explain the theory, a hypothetical illustration is given in Figure 6.17. The hydrophobic and charged groups are involved in substrate binding. Phosphoserine and –SH group of cysteine are involved in catalysis. Lysine and methionine involved in neither substrate binding nor catalysis.
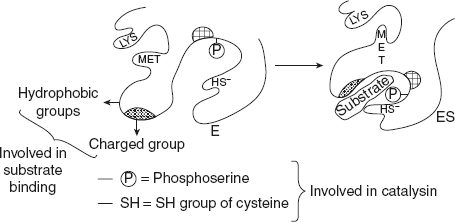
Figure 6.17 Conformational Changes are Brought About by Induced Fit in an Enzyme Molecule
In the absence of substrate, the substrate binding and groups are far apart from each other. But the proximity of substrate induces a conformational change in enzyme molecule, aligning the groups for both substrate binding and catalysis. Simultaneously, the spiral orientation of their groups is also changed so that lysine and methionine are now much closer. Koshland also explains the action of allosteric modulators and competitive inhibition of enzymes.
Leave a Reply