Helmut Beinert first discovered that the iron is present in heme and associated with inorganic sulphur atoms or with sulphur atoms of cysteine residues in the proteins or both. These iron sulphur (Fe-S) centres range from simple structures with single Fe atom coordinated with four cys-SH groups to more complex Fe-S centres with two or four atoms.
Rieske iron sulphur (named after the discoverer) is the iron atom that is coordinated with two of its residues rather than two cysteine groups. All iron-sulphur proteins participate in one electron transfer, among which one iron atom of the iron-sulphur cluster is oxidised or reduced as shown in Figure 8.15. At least eight Fe-S proteins function in mitochondrial electron transfer.
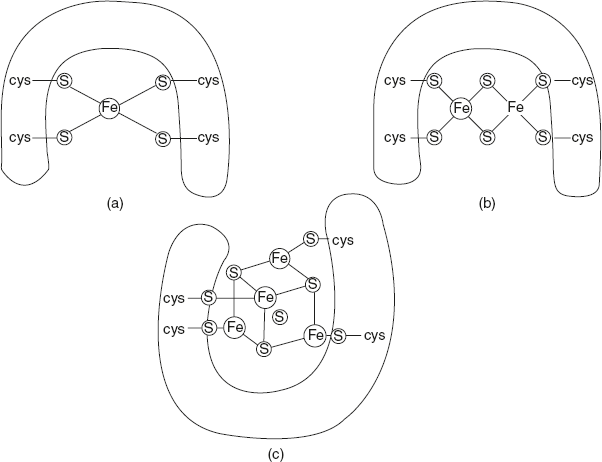
Figure 8.15 Iron-sulphur Centre (a) Single Fe-S Centre (b) Two Fe-2S Centre (c) Four Fe-4S Centre
The electron carriers of the respiratory chain or Electron Transport Chain (ETC) function in multienzyme complexes. Four enzyme complex involved in ETC are the following:
Complex I – NADH dehydrogenase or NADH: Ubiquinone oxidoreductase
Complex II – Succinate dehydrogenase
Complex III – Ubiquinone: Cytochrome C oxidoreductase
Complex IV – Cytochrome oxidase.
Protein components of mitochondrial electron transport chain is given in Table 8.2.
Table 8.2 Protein Components of Mitochondrial Electron Transport Chain
| Enzyme complex | Number of subunits | Prosthetic group |
|---|---|---|
| I NADH DHase | 42 | FMN, Fe-S |
| II Succinate DHase | 5 | FAD, Fe-S |
| III Ubiquinone – cytochrome C oxidoreductase | 11 | Heme, Fe-S |
| IV Cytochrome oxidase | 13 | Heme, Cu A, Cu B |
Complex I NADH to Ubiquione
Complex I also called NADH. Ubiquinone reductase is a large enzyme composed of forty-two different polypeptide chains, including an FMA, containing flavoprotein and at least six iron sulphur centres.
Complex I catalyses two simultaneous and obligately coupled processes:
- Exergonic transfer to ubiquinone of a hydride ion from NADH and a proton from matrix. NADH + H + Q → NAD + QH2
- Endergonic transfer of four protons forms the matrix to the intermembrane space. Complex I is, therefore, a proton pump driven by the energy of electron transfer, and the reaction it catalyses is vectorial. It moves protons in a specific direction from one location (the matrix which becomes negatively charged with the departure of proteins) to another (the intermembrane space which becomes positively charged). The overall reaction is NADH + 5H + Q → NAD + QH2 + 4H+
Figure 8.16 shows that complex I catalyses the transfer of a hydride ion from NADH to FMN, from which two electrons pass through a series of Fe-S centres to the iron-sulphur protein N-2 in the matrix arm of the complex. Electron transfer from N-2 to ubiquinone on the membrane arm forms QH2, which diffuses into the lipid bilayer.
It also drives the expulsion from the matrix of four protons per pair of electrons. This proton flux produces an electrochemical potential across the inner mitochondrial membrane (N-side portion), which conserves some of the energy released by the electron transfer reactions. This electrochemical potential drives ATP synthesis.
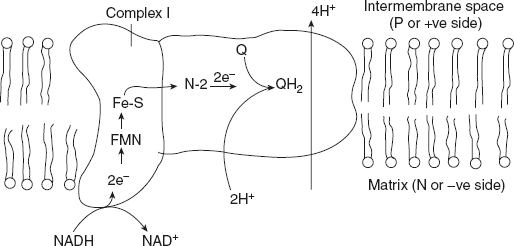
Figure 8.16 NADH-Ubiquinone Oxidoreductase Complex I
Leave a Reply