Allosteric enzymes show relationships between V0 and [S] that differ from Michaelis-Menten kinetics. They do exhibit saturation with the substrate [S] is sufficiently high, but for some allosteric enzymes, when V0 is plotted against [S], a sigmoid saturation curve occurs.
Archibald Hill, in1910, first analysed an equation for the allosteric enzyme kinetics.
For a protein with N binding sites, the equilibrium equation is as follows:
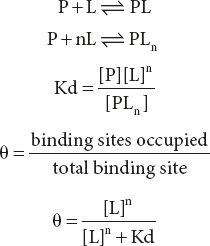
Rearranging, then taking the log of both sides, yields.
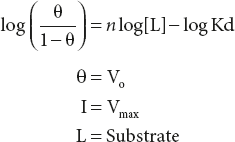
Simply as log
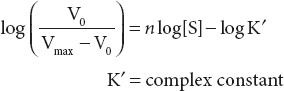
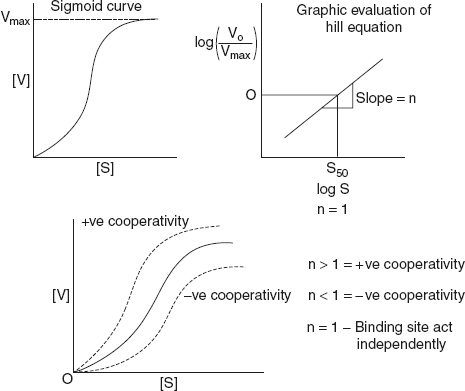
Figure 6.31 Types of Cooperativity Based on the Binding Site of Substrate
The equation states that when [s] is low, compared to k′, the reaction velocity increases as the nth power of [s]. Where n-value depend on the number of substrate binding sites and the type of interactions between these binding sites.
∴ n > l, the sites are cooperative and greater the value of n, the stronger is the cooperativity. n < l, the sites are said to inhibit negative cooperativity as shown in Figure 6.31.
S50 – the concentration of substrate that produces half maximal velocity drops a perpendicular line to x-axis from the point where log (V0/Vmax − V0) = 0.
Leave a Reply