- Allopurinol first acts as a substrate and as an inhibitor of the enzyme xanthine oxidase.
- The enzyme hydroxylate allopurinol to alloxanthine, which then remains tightly bound to the active site of the enzyme.
- The molybdenum atom of the enzyme is kept tightly bound with the alloxanthine in the +4 oxidation state, and thus the normal +6 oxidation state of molybdenum, which is necessary for the catalytic activity of the enzyme, is not restored. Thus, the product uric acid is not formed.
(This mode of action of allopurinol is an example of suicide inhibition, in which an enzyme converts a compound into a potent inhibitor that immediately inactivates the enzyme.)
- Gout is a disease characterised by gout pain and swelling in joints due to excessive deportation of sodium water, crystals in joints, subcutaneous tissues, and so on.
- Allopurinol alternates the symptoms of gout by decreasing the rate of uric acid production while increasing the levels of the more solute hypoxanthine and xanthine.
- Anticancer Agents/Chemotherapy
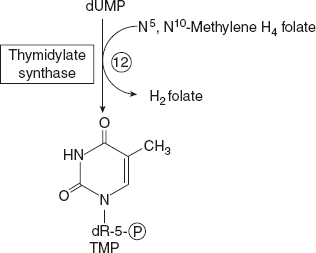
- dTMP is synthesised from dUMP by thymidylate synthase with N5, N10methylenetetrahydrofolate as the methyl donor.
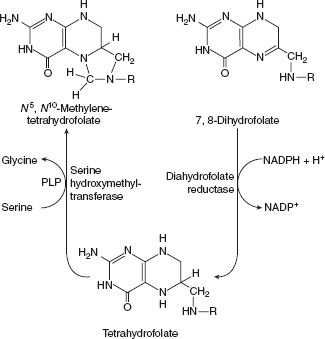
- Dihydrofolate reductase catalysed the reduction of DHF to THF by NADPH.
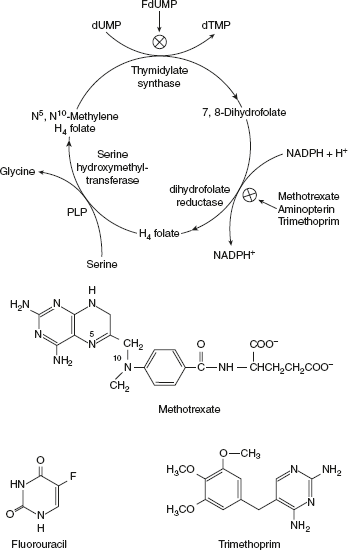
- Dihydrofolate reductase is very strongly competitively inhibited by methotrexate, which is an analog of FH2 (DHF); another is an important folic acid antagonist.
- Methotrexate, amino protein, and trimethoprim are all DHF analogs that completes, although nearly irreversibly bind to reductase, with a 1000-fold greater affinity than DHF does.
- These antifolates which reduce DHFR prevents dTMP synthesis. Rapidly proliferating cells as cancer cells require a steady supply of dTMP in order to survive. Thus, methotrexate is used as an effective anticancer agent, particularly against childhood leukaemia.
- AntibioticsFolic acid is a coenzyme used in certain biosynthetic reaction although it is not synthesised in man. Some bacteria synthesise it using para aminobenzoic acid (PABA). PABA → Folic acid
(Sulphonamide)Sulphonamide are chemically and physically similar to PABA and can competitively inhibit the synthesis of folic acid (an essential vitamin for microbes), thus, leading to the inhibition of the growth of susceptible organism.Sulphonamide can be toxic to micro-organism and virtually harmless to the host as the host obtains its folic acid sequence from the diet and not from PABA.
- Treatment of Ethylene Glycol Poisoning
- About fifty deaths occur annually from the ingestion of ethylene glycol, a constituent of permanent-type automobile anti-freeze.
- Ethylene glycol itself is not lethally toxic; rather, the harm is done by oxalic acid, an oxidation product of ethylene glycol.
- The first step is the conversion as the oxidation of ethylene glycol by alcohol dehydrogenase.
- This reaction can be effectively inhibited by the administration of a nearly intoxicating dose of ethanol, which acts as a competitive inhibitor of alcohol dehydrogenase.
- The ethylene glycol is then excreted harmlessly. (The same rationale is used for the therapy of methanol poisoning.)
- Treatment of Hypotensive Shock
- Competitive inhibition explains the action of several other drugs. For example, ephedrine and amaphetamine prolong the effect of the hormones like adrenaline/noradrenaline, and 5-hydroxytryptamine by competitively inhibiting the enzyme monoamine oxidase (MAO), which brings about the oxidative deamination of the above-mentioned hormones.
- A similar explanation has been put forward for the action of cocaine and anti-depressive drugs like proniazid and tranylcypromine.



Leave a Reply