Liquefied gases have been used widely as propellants for most of the aerosol products. In a sealed container such as an aerosol can, the liquefied gas exists in two phases—liquid and gas, behaving as a two-phase system. The molecules in the vapor stage are responsible for exerting the pressure on the contents. As the number of molecules in the vapor phase increases, vapor pressure of the propellant also increases proportionately. The pressure attained at equilibrium is known as the vapor pressure and is unique for a given propellant at a given time. This vapor pressure is exerted equally in all directions and is independent of the quantity present.
During valve actuation, the vapor pressure exerted by the gas on the liquid phase is responsible for pushing the product up (liquid state) through the dip tube. In cases where there is no dip tube (e.g. MDIs), the container is used in the inverted position. When the valve is opened, the liquid phase is expelled with sudden volume of expansion and comes in contact with the atmospheric temperature. The propellant, whose boiling point is much less than the room temperature, vaporizes immediately. To restore the drop in pressure inside the container, more of the liquid propellant vaporizes into the head space. In comparison with compressed gases, one advantage with these propellants is that there is no drop in pressure inside the container during the usage of the product. The pressure exerted is independent of the propellant concentration within the container. Due to its large volume expansion ratio, the spray performance is maintained constant throughout the life of the aerosol until its last dose of the drug delivery.
Comparative diagram of aerosols with liquefied propellant and compressed gas propellant is shown in Figure 12.15.
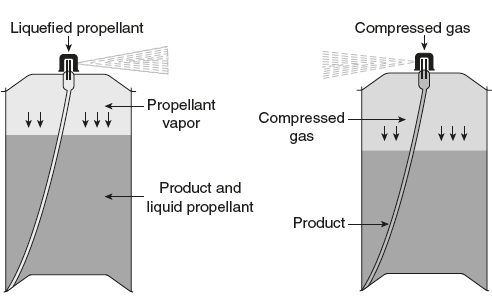
Figure 12.15 Comparative Diagram of Aerosols with Liquefied Propellant and Compressed Gas Propellant
Examples for fluorocarbon propellants used in aerosols are trichloromonofluoromethane (propellant 11), Dichlorodifluoromethane (propellant 12), and dichlorotetrafluoroethane (propellant 114). The three-digit numerical designation is used to identify each propellant. The first digit is one less than the number of carbon atom in the compound. The second digit is one more than the number of hydrogen atoms in the compound. The last digit represents the actual number of fluorine atoms. If the value of first digit numerical designation is zero, it can be represented by only two digits. The remaining carbon valency should be satisfied by chlorine atom.
| Examples: | Propellant 11 is CFCl3 |
| Propellant 12 is CF2Cl2 | |
| Propellant 114 is C2F4Cl2 |
Leave a Reply