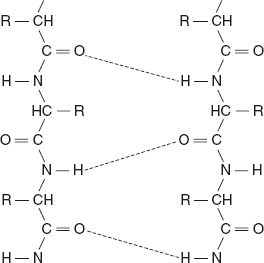
The polypeptide chains are arranged in the same direction if the N-terminal ends of all the participating polypeptide chains lie on the same edge of the sheet with all C-terminal ends on the opposite edge. In other words, the hydrogen-bonded neighbouring polypeptides are aligned in the same direction of the N— to —C terminals. Parallel β-pleated sheet structure is given below.
Anti-parallel β-Pleated Sheets
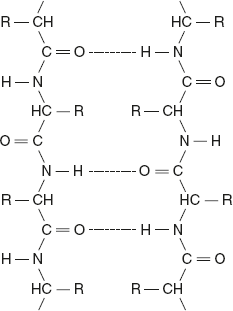
The chains run in opposite direction; that is, the hydrogen-bonded neighbouring polypeptides are aligned in opposite direction. Collinear hydrogen bonds are formed in anti-parallel β-sheets, which make them more stable than the parallel β-sheets.
Silk fibroin is one example of a protein that has anti-parallel pleated sheet structure. Anti-parallel β-pleated sheet structure is as given below.
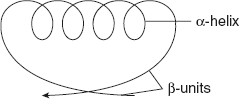
Mixed parallel and anti-parallel β-sheets are observed in many proteins. These proteins contain the combined α-helix and β-pleated sheet structure. This is called a super secondary structure. In β α β units, α-helix is connected to two β-pleated sheets.
In the β-meander structure, two anti-parallel β-sheets are joined by polar amino acids and glycine to produce a polypeptide chain called β-turns, as shown in Figure 3.6.
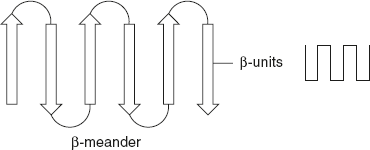
Figure 3.6 β-Meander Pattern
In α-α units, two structure α-helices are separated by a loop or non-helical segment as shown in Figure 3.7.
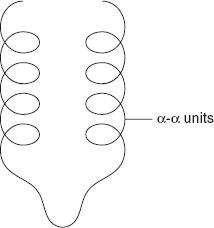
Figure 3.7 α–α Units Pattern
β-barrel arrangement is formed when various β-sheet configurations fold back on themselves. β-barrel arrangement is formed when various β-sheet configurations fold back on themselves as shown in Figure 3.8.
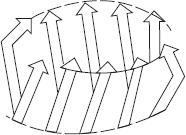
Figure 3.8 β-Barrel Arrangement
A Greek pottery pattern design contains an anti-parallel β-sheet folded back on itself and resembles the Greek key, which is shown in Figure 3.9
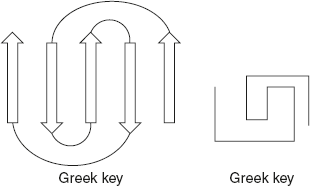
Figure 3.9 Greek Key Pattern
Leave a Reply