PFK-1 catalysed step is the irreversible step that commits a cell to convert glucose to glycolysis. Apart from the substrate-binding sites, this enzyme contains additional sites (regulatory sites) where allosteric activators or inhibitors bind. ATP not only serves as a substrate for PFK-1 but it also becomes the end product of the glycolytic pathway. When the concentration of ATP is more in the cells, it inhibits PFK-1 by binding to an allosteric site and lowering the affinity of the enzyme for fructose 6-phosphate. ADP and AMP concentration increases and they act allosterically to relieve this inhibition of ATP production. Thus a high level of ADP and AMP activates the enzyme, whereas increase in ATP level lowers the activity of PFK-1.
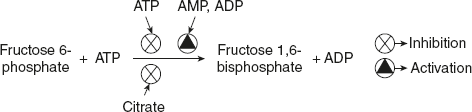
Citrate also acts as an allosteric regulator of PFK-1, which is produced as the key intermediate in the aerobic oxidation of pyruvate, fatty acids, and amino acids. When the concentration of citrate increases, the ATP concentration decreases; as a result, it inhibits PFK-1 and thereby further reduces the flow of glucose through the glycolysis pathway. Allosteric inhibition of PFK-1 by ATP is shown by substrate activity curve as shown in Figure 8.7.
At low ATP, the K0.5 for fructose 6-phosphate is relatively low, enabling the enzyme to function at a high rate at relatively low concentrations of fructose 6-phosphate.
Leave a Reply