The use of galenicals or plant extracts directly for therapeutic purpose in modern medicine stems from the herbal remedies of the Middle Ages. In addition to this such extracts may provide the first stage in the isolation of active ingredients. With the resurgence of interest in herbal medicine several of these plant extracts have regained their popular usage as medicinals. Today a significant percentage of medicinal plant material is used to make plant extracts. This is carried out either by the end product manufacturer or by extract companies. The current trend of medicinal plant-based drug industry is to procure standardized extracts of plants as raw material. Plant extracts could be standardized fluid/solid extracts, powders or tinctures. Standardized extracts of many plants such as Aloe sps, Atropa belladonna, Cassia angustifolia, Capsicum annum, Centella asiatica, Cephaelis ipecacuanha, Digitalis sps, Commiphora mukul, Panax ginseng, etc., are widely used in health care.
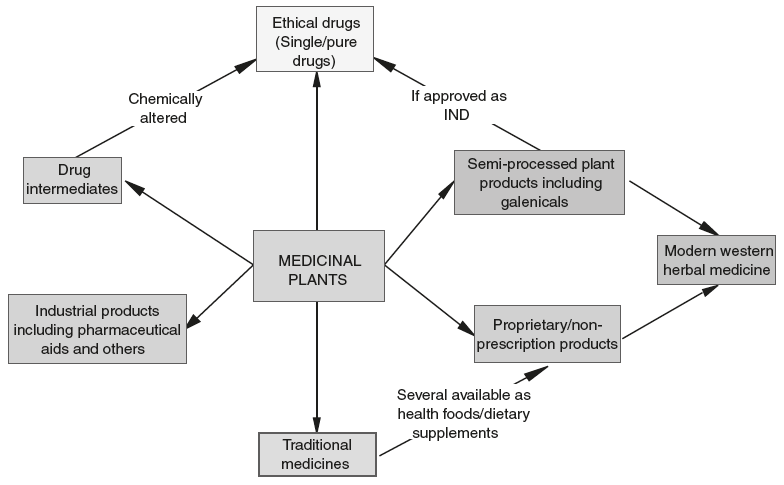
Figure 3.1. Medicinal plant products traded – their inter-relationship
An important factor behind the increasing use of standardized extracts is that they can be better incorporated into modern dosage forms and also standardization is considered necessary to achieve as much control in double blind studies as possible.
The following are the types of plant extracts or processed plant products in trade:
- Dried whole/plant parts size-reduced to form powders;
- Fluid extracts using different solvents resulting in tinctures, medicinal wines, syrups, liquid mixtures etc.;
- Solid extracts standardized and formulated or fractionated to yield concentrates of active ingredients or pure compounds.
Standardized plant extracts are sold as over-the-counter (OTC), non-prescription items. According to International Medical Statistics (IMS), half of the world expenditure on branded non-prescription herbal medicines in 1994 (US $11.9 billion) was spent in Europe. Such decoctions, tinctures and other galenicals form a part of many pharmacopoeias of the world.
A conspicuous feature of the European OTC market is that for many of the products, the available evidence of clinical efficacy is still inconclusive.
Sales of plant extracts is however undoubtedly increasing evidenced by the growth of one of Europe’s leading extract suppliers, Indena, which increased its operating revenue by 92% from 1991 to 1994 (according to F & S data base compiled by Frost & Sullivan Publishers, London). More recently several extract companies have been set up in the Far East in an attempt to increase the value of the raw material through processing. For e.g., Qingdao Huanzhong Pharmaceutical Ltd is a Sino-Japanese joint venture in China with a production capacity of 240 tonnes of extracts destined for export to Japan and other international markets. Another example is that of Southern Herbals in India which started production of plant extracts in 1992 and is reported to be supplying companies such as Amgen, Bristol Meyer Squibb and Fujisawa. Improved methods for the processing of medicinal and aromatic plants and new techniques for quality assessment are being developed rapidly to keep up with recent developments and new international requirements. Super critical fluid extraction is a recent alternative for solvent extraction. It enables effective and quick processing of phytopharmaceuticals and aids complete removal of residual pesticides, toxins and surfactants in them.
Leave a Reply