Physical Properties
They are insoluble in water but are readily soluble in fat solvents like alcohol, ether, chloroform, and so on.
They are greasy to touch and leave an oily impression on paper.
Pure glycerides are tasteless, odourless, and colourless.
Fats used in food have specific flavour and colour. The flavour is attributed to the presence of certain foreign substances absorbed by the fat from its natural environment during the processing of the fat; for example, the flavour of butter is due to the presence of bacterial flora, which is carefully controlled to impart special flavour to butter.
The quantity of saturated and unsaturated fatty acids present in the fat determines its hardness. For example, fat containing saturated fatty acids will be solid in nature at room temperature, whereas fat containing unsaturated fatty acids, for example oils, will be liquid in nature at room temperature.
Fats possess definite melting points. The melting point of fat is usually higher than the temperature at which it solidifies. The melting points of natural fats are not sharp because they are mixtures of several fats.Example:Melting point of beef fat is 49.5°C. Solidification temperature of beef fat is 36°C.
Fats have lesser specific gravity than water and, therefore, float in water. The specific gravity of solid fat is less than liquid fat. Specific gravity of solid fat is 0.86. Specific gravity of liquid fat is 0.91.
Spreading of fat: When liquid fat is placed on water, it spreads uniformly over the surface of water. The effect of this property is to lower the surface tension and help the transport of fat. The phenomenon of spreading is explained as follows:Two groups in the structure of fats exert their role in the mechanism of spreading. These are the following:- A hydrophilic carboxyl group, which dissolves in water and is referred as C1.
- A hydrophobic hydrocarbon chain, which forms a layer on the surface and is referred as C2 which is shown in Figure 4.3.
Thus, CH3COOH, a fatty acid, contains the hydrocarbon chain (CH3), which is referred as C2 and carboxyl group (COOH), represented as C1. The two groups are arranged above and below the surface of water.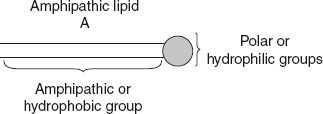 Figure 4.3 Diagrammatic Representation of Fat Structure
Figure 4.3 Diagrammatic Representation of Fat Structure
Emulsification: Fats are insoluble in water; they can be broken into minute droplets and dispersed in water. This is called emulsification as shown in Figure 4.4. This greatly increases the surface area of fats, and this is an essential requisite for digestion of fats in the intestines. Emulsification is brought about by the following:
- Mechanical action: This can be facilitated by mixing mechanically or passing superheated steam.
- Chemical action: This is accomplished by the action of bile salts, which lower the surface tension.
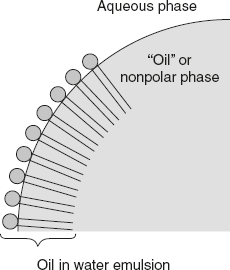 Figure 4.4 Diagrammatic Representation of Emulsion
Figure 4.4 Diagrammatic Representation of Emulsion
Leave a Reply