A fourth degree of complexity in protein structure has been recognised, which is known as quaternary structure. High molecular weight proteins are composed of several polypeptide chains. Each polypeptide components is called subunit. Subunits in a protein complex may be identical or quite different. The identical peptides in a polypeptide are referred to as oligomers. Mostly oligomers contain two or four subunits referred to as dimers and tetramers. Synthesis of individual subunits is more efficient than the lengthy polypeptide because in supra molecular structures such as collagen, replacement of worn-out or damaged components can be managed more efficiently.
Quaternary structure is stabilised by non-covalent interactions such as hydrophobic interactions, electrostatic interactions, and hydrogen bonds.
During protein folding, hydrophilic interactions are very important because the structure of complementary interfacing surfaces is the same as those observed in the interior globular protein domains.
Example of quaternary structure is globular protein – haemoglobin. Haemoglobin represented as Hb— is a roughly spherical molecule found in RBC where its primary function is to transport O2 from lungs to every tissue in the body. Its tetramer protein contains four polypeptide chains. The four chains are held together by non-covalent interactions. Each chain contains a haem group and a single O2 binding site as shown in Figure 3.13.
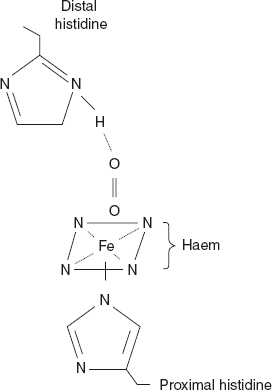
Figure 3.13 O2 Binding Site of Haem Created by a Folded Globin Chain
Haemoglobin A1, the principle Hb in adults, consists of two alpha (α) chains and two beta (β) chains. Adults also have delta (δ) chains in place of two beta (β) chains of Hb A2 in α2δ2.
The first Hb appearing during embryonic development has two subunits ζ2ε2; the two zeta (ζ) chains are analogous to the α-chains and the two epsilon (ε) chains are analogous to the β-chains.
When after about six weeks, ζ chain production increases, the tetramer α2 ε2 appears; that is, ζ is replaced by α. Third embryonic haemoglobin is ζ2 γ2, where ε is replaced by γ. These last two Hb represent transition phases, leading to the appearance of fetal haemoglobin (Hb F), whose tetrameric composition is α2 γ2. The α and ζ chains contain 141 residues each. The β, γ, and δ chains contain 146 residues each have homologous amino acid sequence as shown in Figure 3.14. The γ and δ chains differ from the β chain at 39 and 10 amino acid residues.
Human Hb protein consists of four polypeptide chains of two types; two α-chains is two β-chains. The polypeptide portion is collectively called globin.
The α-chain has valine at the N-terminal and arginine at the C-terminal, whereas in the β-chain, valine in situated at the N-terminal and histidine at the C-terminal.
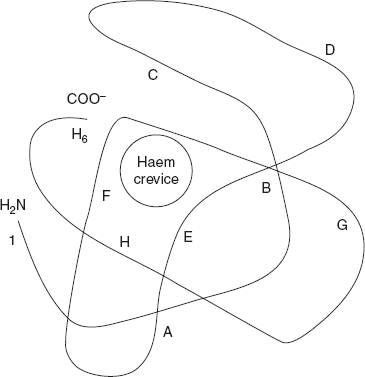
Figure 3.14 Structure of Single β-Chain of Haemoglobin
The α-chains are in contact with the β-chains. Apart from this contact, there are also interactions between the two α-chains and the two β-chains. The haem prosthetic group fits into the crevice near the exterior of the globin chain. O2 binds to the haem groups.
The α-chain contains 141 residues and the β-chain contains 146 residues. So, totally there are 574 (141 × 2 + 146 × 2 = 574) amino acid residues. Individual α-chains and β-chains have a characteristic tertiary structure, in which the chain is folded.
The bond between the α-chains and the β-chains are ionic bonds and hydrogen bonds. The two pairs of chain are joined by ionic, hydrogen, and hydrophobic forces. Thus four polypeptides are joined together into tetrahedral structure and form a quaternary structure, as shown in Figure 3.15.
The haem group is occupied in a pocket lined with hydrophobic R—groups. It is fixed to its polypeptide chain through a coordination bond of the iron group to the R—group of histidine residue.
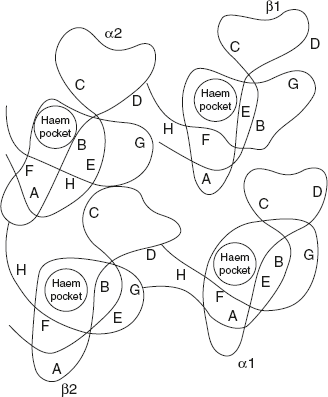
Figure 3.15 Quaternary Structure of Haemoglobin
Leave a Reply